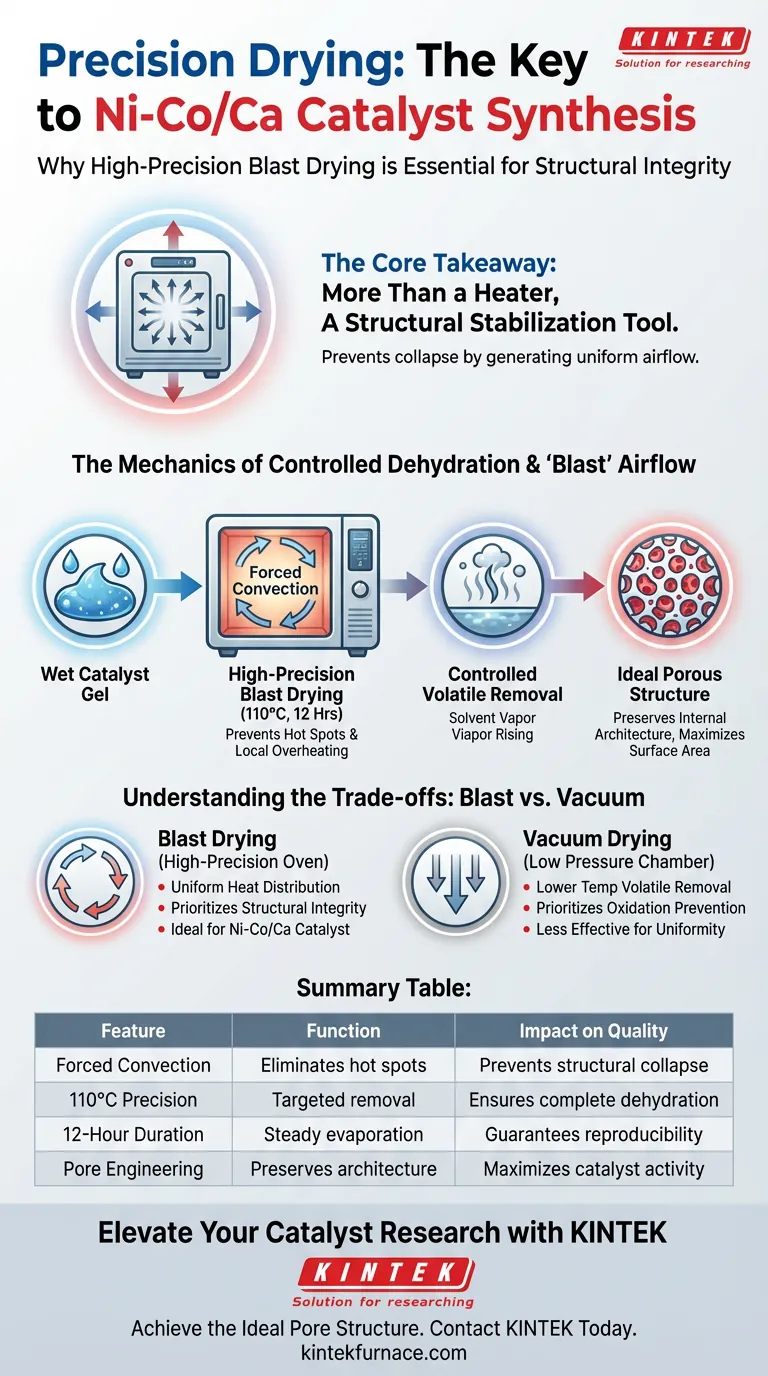
Precision airflow is the defining factor that separates successful catalyst synthesis from structural failure. A high-precision blast drying oven is utilized during the Ni-Co/Ca catalyst preparation to strictly control the dehydration environment, specifically subjecting the gel to 110°C for 12 hours. This process ensures the complete removal of physically adsorbed water and residual solvents without compromising the material's internal architecture.
The Core Takeaway The blast drying oven is not merely a heating device; it is a structural stabilization tool. Its primary function is to generate uniform airflow that prevents local overheating, thereby protecting the catalyst gel from structural collapse during the critical transition from wet gel to solid porous material.
The Mechanics of Controlled Dehydration
Removing Volatiles Without Damage
The preparation of Ni-Co/Ca catalysts involves a catalyst gel that is saturated with moisture and solvents.
To prepare this material for calcination, it must be treated at 110°C for a duration of 12 hours.
This specific time-temperature profile is calculated to thoroughly eliminate physically adsorbed water and any residual solvents trapped within the gel's pores.
The Role of Precision Control
Standard drying methods often result in uneven evaporation rates.
A high-precision oven maintains tight thermal stability, ensuring that the solvent removal occurs at a steady, predicted rate.
This controlled evaporation is vital for preventing rapid shrinkage or cracking that often occurs when solvents exit the material too quickly or unevenly.
Why "Blast" Airflow is Critical
Preventing Local Overheating
The "blast" feature refers to forced convection—mechanically circulating air throughout the chamber.
Without this circulation, stagnant air pockets can create "hot spots" where temperatures exceed the set point.
In catalyst preparation, local overheating causes structural collapse. The blast oven eliminates this risk by ensuring the thermal load is distributed perfectly evenly across the sample.
Establishing the Ideal Pore Structure
The ultimate goal of this drying phase is pore engineering.
By preventing collapse during dehydration, the oven preserves the delicate framework of the gel.
This establishes the ideal pore structure, which provides the necessary surface area and accessibility for the catalyst to function effectively in subsequent chemical reactions.
Understanding the Trade-offs
Blast Drying vs. Vacuum Drying
While blast drying excels at creating structural uniformity through airflow, it is distinct from vacuum drying.
Vacuum drying lowers pressure to remove solvents at lower temperatures (often below 100°C), which is preferable for materials that are highly sensitive to oxidation or thermal decomposition.
However, vacuum drying lacks the convective airflow of a blast oven. For the Ni-Co/Ca catalyst, the priority is preventing structural collapse via uniform heat distribution, making the blast oven the superior choice over vacuum methods for this specific phase.
The Risk of Improper Airflow
If the airflow is too aggressive or the temperature ramp is uncontrolled, you risk creating an "egg-shell" effect where the outer layer dries and hardens while the inside remains wet.
High-precision blast ovens are designed to mitigate this by maintaining a balance between efficient heat transfer and gentle solvent removal.
Making the Right Choice for Your Goal
To ensure you are applying this equipment correctly for your specific catalyst requirements:
- If your primary focus is structural integrity: Prioritize the high-precision blast oven to ensure uniform airflow and prevent pore collapse during dehydration.
- If your primary focus is oxidation prevention: Consider vacuum drying alternatives only if the active components are liable to decompose in the presence of oxygen at 110°C.
- If your primary focus is reproducibility: strictly adhere to the 12-hour duration to guarantee that all physically adsorbed water is removed consistently across every batch.
The blast drying oven transforms a simple drying step into a critical quality control measure for catalyst morphology.
Summary Table:
| Feature | Function in Catalyst Preparation | Impact on Quality |
|---|---|---|
| Forced Convection | Eliminates hot spots via uniform airflow | Prevents structural collapse/pore damage |
| 110°C Precision | Targeted removal of volatiles | Ensures complete dehydration of the gel |
| 12-Hour Duration | Steady solvent evaporation | Guarantees batch-to-batch reproducibility |
| Pore Engineering | Preserves internal architecture | Maximizes catalyst surface area and activity |
Elevate Your Catalyst Research with KINTEK
Don't let uneven drying compromise your material's architecture. KINTEK provides high-precision drying solutions designed for the rigorous demands of catalyst synthesis. Backed by expert R&D and manufacturing, we offer a full range of lab equipment—including high-temp furnaces, Muffle, Tube, and Vacuum systems—all customizable for your unique research needs.
Ready to achieve the ideal pore structure? Contact KINTEK Today to Consult an Expert
Visual Guide
References
- Jiaxiang Wang, Yueyao Li. Investigating the Catalytic Influence of Boron on Ni-Co/Ca Catalysts for Improved Syngas Generation from Rice Straw Pyrolysis. DOI: 10.3390/molecules29081730
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- How does a circulating mineral oil jacket heating system function? Ensure Precision in Wood Thermal Modification
- What role does Iodine (I2) play as a transport agent in CVT for FexTaSe2? Unlock Efficient Single Crystal Growth
- What PPE is suggested for adjusting controls or handling equipment during furnace operation? Essential Gear for Operator Safety
- What are the advantages of PVD equipment for solar absorber films? Achieve Nanometer Precision and Maximum Efficiency
- Why is an industrial forced air drying oven required for drying banana slices? Unlock Precision & Nutritional Quality
- How should materials with high moisture content be handled before heating? Ensure Safety and Quality in Thermal Processing
- What are the main types of furnaces used in foundries for metal casting? Choose the Best for Your Metal
- Why is an industrial drying oven necessary for Boron Carbide mixed slurries? Ensure Coating Integrity & Precision



















