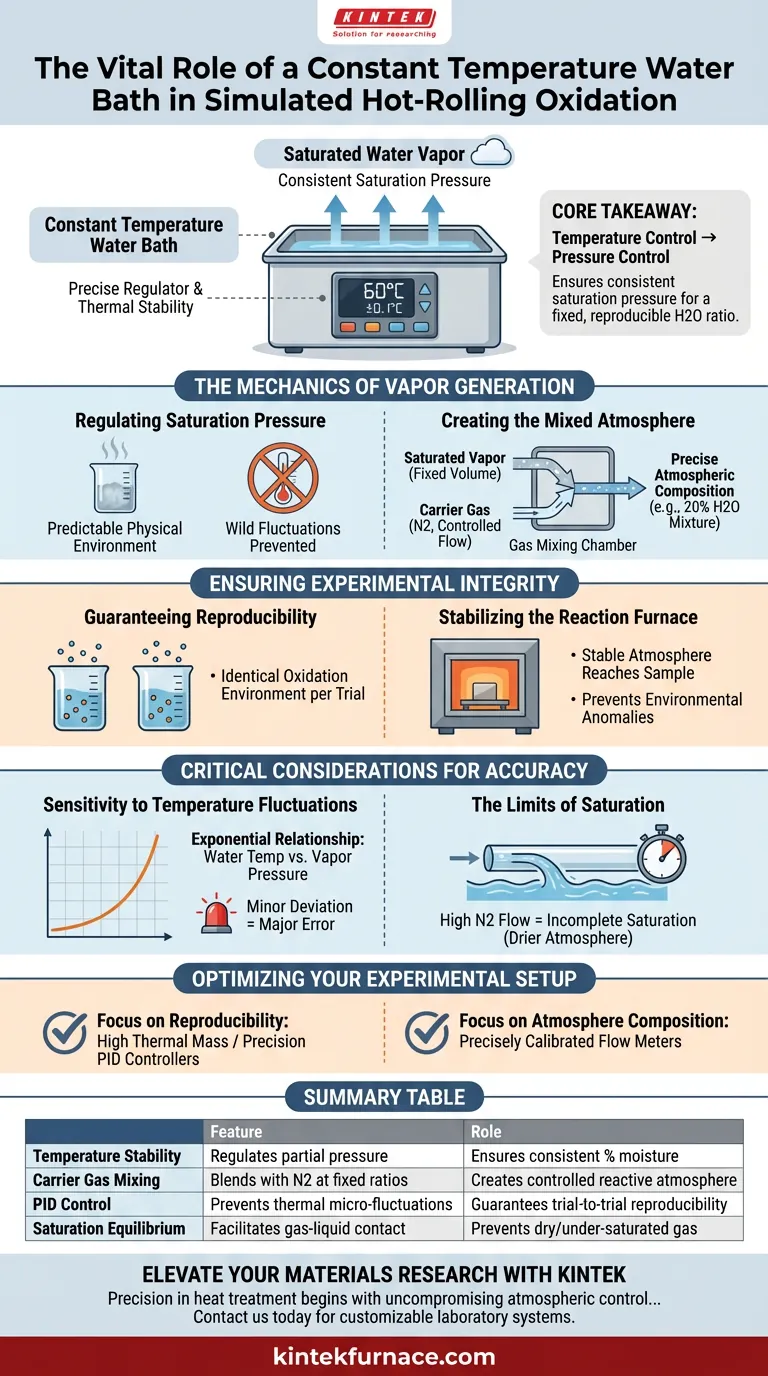
The constant temperature water bath acts as the precise regulator for humidity levels in oxidation experiments. By maintaining the water source at a specific, unwavering temperature (such as 60°C), it dictates the exact volume of saturated water vapor produced. This thermal stability is the foundation for creating a controlled experimental atmosphere.
Core Takeaway In simulated hot-rolling oxidation, the water bath converts temperature control into pressure control. By locking the water temperature, the system ensures a consistent saturation pressure, enabling the delivery of a fixed, reproducible ratio of water vapor (e.g., 20% H2O) when mixed with a carrier gas.
The Mechanics of Vapor Generation
Regulating Saturation Pressure
The fundamental role of the water bath is to create a predictable physical environment. By heating the water container to a precise setpoint, the bath ensures the water generates saturated vapor at a specific partial pressure.
Without this constant temperature, the amount of steam generated would fluctuate wildly. This would make it impossible to calculate or control the specific humidity entering the reaction chamber.
Creating the Mixed Atmosphere
The vapor generated by the water bath does not act alone; it is designed to work in tandem with a carrier gas, typically Nitrogen ($N_2$).
The system combines the steady stream of saturated vapor with a controlled flow of nitrogen. Because the vapor volume is fixed by the bath's temperature, adjusting the carrier gas flow allows the researcher to dial in a specific atmospheric composition, such as a 20% water vapor mixture.
Ensuring Experimental Integrity
Guaranteeing Reproducibility
Simulated hot-rolling oxidation tests require data that can be compared across different trials. The constant temperature bath ensures that the oxidation environment remains identical from one experiment to the next.
Stabilizing the Reaction Furnace
The mixture delivered by the system enters the reaction furnace where the hot-rolling simulation occurs.
The water bath ensures that the atmosphere reaching the sample is stable. This prevents environmental anomalies from skewing the data regarding how the metal oxidizes under heat and stress.
Critical Considerations for Accuracy
Sensitivity to Temperature Fluctuations
It is vital to understand that the relationship between water temperature and vapor pressure is exponential, not linear.
Even a minor deviation in the water bath's temperature can cause a disproportionately large error in the moisture content of the gas. Therefore, the bath's ability to hold a tight tolerance is more critical than the setpoint itself.
The Limits of Saturation
The system assumes that the carrier gas becomes fully saturated with water vapor as it passes through the container.
If the flow rate of the nitrogen carrier gas is too high, it may not have enough residence time to reach equilibrium with the water. This results in an atmosphere that is drier than calculated, regardless of the water bath's temperature setting.
Optimizing Your Experimental Setup
To ensure accurate simulation of hot-rolling oxidation, align your equipment settings with your specific experimental goals:
- If your primary focus is Reproducibility: Ensure your water bath has high thermal mass or precision PID controllers to prevent micro-fluctuations in temperature.
- If your primary focus is Atmosphere Composition: Calibrate your carrier gas flow meters precisely, as they work in direct proportion with the vapor pressure generated by the bath to determine the final ratio.
Precision in the water bath serves as the control variable that makes accurate oxidation simulation possible.
Summary Table:
| Feature | Role in Oxidation Simulation | Impact on Experimental Accuracy |
|---|---|---|
| Temperature Stability | Regulates saturated vapor partial pressure | Ensures consistent moisture content (%) |
| Carrier Gas Mixing | Blends vapor with $N_2$ at fixed ratios | Creates a controlled reactive atmosphere |
| PID Control | Prevents thermal micro-fluctuations | Guarantees trial-to-trial reproducibility |
| Saturation Equilibrium | Facilitates gas-liquid phase contact | Prevents dry/under-saturated gas delivery |
Elevate Your Materials Research with KINTEK
Precision in heat treatment begins with uncompromising atmospheric control. At KINTEK, we understand that even a minor temperature deviation can compromise your oxidation data. Backed by expert R&D and manufacturing, we provide high-performance solutions including Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which can be customized for your unique high-temperature laboratory needs.
Whether you are simulating hot-rolling processes or conducting complex chemical vapor depositions, our furnaces deliver the thermal stability your research demands. Contact us today to discuss how our customizable laboratory systems can enhance your experimental accuracy and efficiency.
Visual Guide
References
- Seksan Singthanu, Thanasak Nilsonthi. A Comparative Study of the Oxidation Behavior of Hot-Rolled Steel established from Medium and Thin Slabs oxidized in 20% H2O-N2 at 600-900°C. DOI: 10.48084/etasr.6168
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- How does a reactor system control chlorine sources in oxychlorination? Master Catalyst Regeneration Control
- What are some examples of medium-temperature industrial heating processes? Optimize Material Properties Efficiently
- What are the advantages of using electron bombardment heating systems for Niobium? Achieve Industrial Fidelity
- Why are reactive polyurethane systems a focus of thermal analysis in leather finishing? Balance Safety and Aesthetics
- What is the primary function of a high vacuum drying oven in B4C/Al powder pretreatment? Protect Purity & Prevent Pores
- How is a precision micro-Raman spectrometer utilized in the characterization of SSBSN ceramics? Master Phase Verification
- What is the function of the annealing furnace? Strategically Control Material Properties for Reliability
- Why is high-precision constant temperature heating equipment required when preparing 17-4 PH stainless steel composite?



















