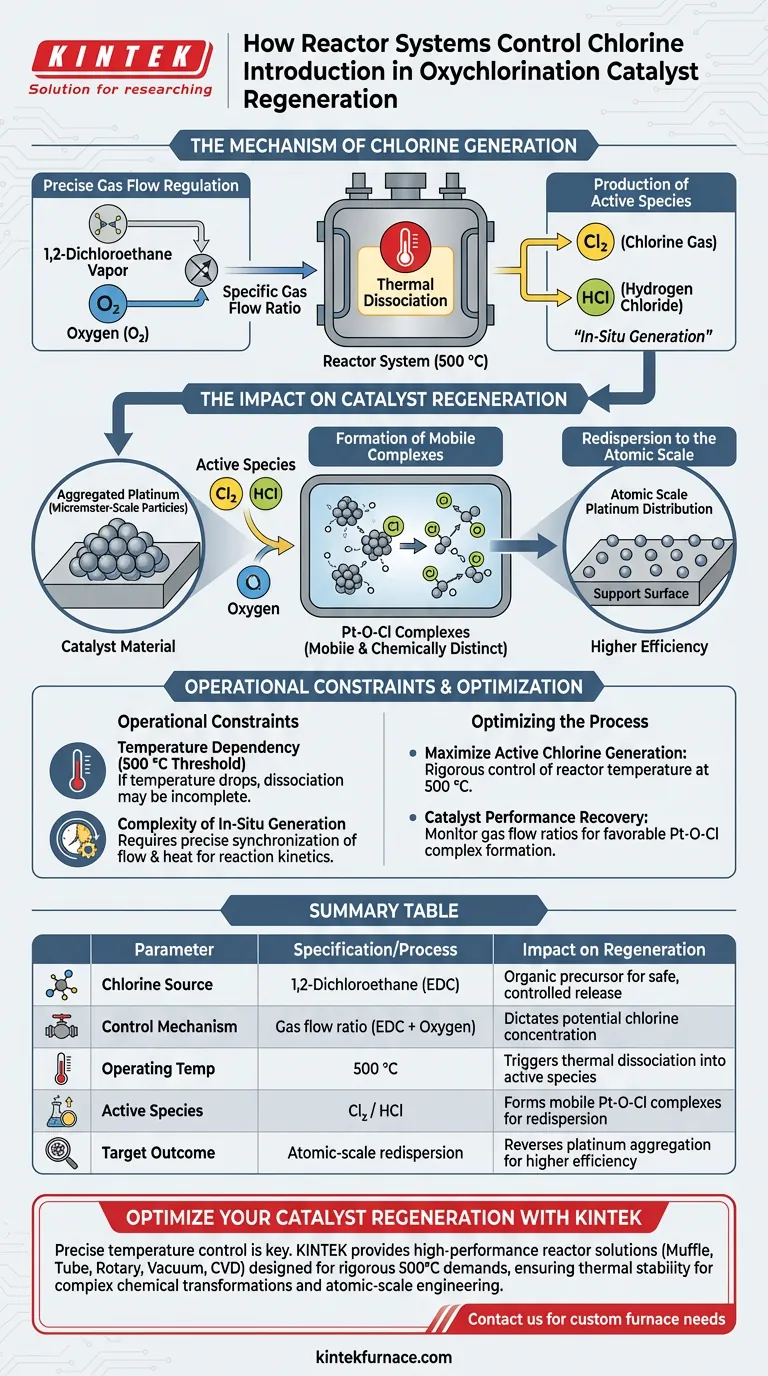
The reactor system controls the introduction of chlorine by precisely regulating the gas flow ratio of 1,2-dichloroethane vapor mixed with oxygen. Rather than injecting active chlorine directly, the system uses this organic compound as a precursor, which releases chlorine species only when subjected to specific thermal conditions within the reactor.
Core Takeaway The system relies on the in-situ generation of chlorine species through the thermal dissociation of 1,2-dichloroethane at 500 °C. This controlled release is critical for creating the specific chemical environment—specifically Pt-O-Cl complexes—required to redisperse aggregated platinum particles back to an atomic scale.

The Mechanism of Chlorine Generation
The control system does not manage a simple flow of chlorine gas; it manages a chemical transformation. The process is defined by the conversion of a stable precursor into active chemical agents.
Precise Gas Flow Regulation
The primary control lever is the gas flow ratio. The system creates a specific mixture of 1,2-dichloroethane vapor and oxygen.
By adjusting this ratio, the system dictates the potential concentration of chlorine available for the regeneration process.
Thermal Dissociation
The reactor acts as the site for thermal breakdown. The system maintains an operating temperature of 500 °C.
At this temperature, the 1,2-dichloroethane chemically dissociates. This breakdown is the mechanism that effectively "introduces" the chlorine into the reaction environment.
Production of Active Species
The dissociation process yields active chlorine species, specifically Cl2 (chlorine gas) or HCl (hydrogen chloride).
These are the agents capable of interacting with the catalyst material. The system controls their production rate indirectly by managing the precursor flow and reactor temperature.
The Impact on Catalyst Regeneration
The introduction of chlorine is not an end in itself; it is the means to reverse catalyst degradation. The goal is to alter the physical state of platinum particles.
Targeting Aggregated Platinum
Over time, platinum particles on a catalyst may clump together, forming aggregated micrometer-scale particles.
The active chlorine species generated by the reactor interact directly with these aggregates.
Formation of Mobile Complexes
The reaction between the active chlorine, oxygen, and the platinum aggregates forms Pt-O-Cl complexes.
These complexes are chemically distinct from pure platinum. Crucially, they are mobile, meaning they can move across the support surface.
Redispersion to the Atomic Scale
The formation of these mobile complexes provides the necessary physicochemical conditions for redispersion.
This allows the platinum to transition from large, ineffective micrometer-sized clumps back to a highly efficient atomic scale distribution.
Understanding the Operational Constraints
While effective, this method of chlorine introduction relies on strict process parameters. Deviations can compromise the regeneration cycle.
Temperature Dependency
The system is heavily dependent on maintaining the 500 °C threshold.
If the temperature drops, the dissociation of 1,2-dichloroethane may become incomplete, failing to produce sufficient active chlorine species for the reaction.
Complexity of In-Situ Generation
Unlike direct chlorine injection, this process requires the simultaneous management of dissociation and reaction.
The system must ensure that the generated active species (Cl2 or HCl) are produced at a rate that matches the kinetics required to form Pt-O-Cl complexes, requiring precise synchronization of flow and heat.
Optimizing the Regeneration Process
To ensure successful catalyst redispersion, you must focus on the variables that drive the chemical transformation of the precursor.
- If your primary focus is maximizing active chlorine generation: rigorous control of the reactor temperature at 500 °C is essential to ensure full dissociation of the 1,2-dichloroethane.
- If your primary focus is catalyst performance recovery: monitor the gas flow ratios to ensure the stoichiometry favors the formation of mobile Pt-O-Cl complexes, which are required to break down micrometer-scale aggregates.
By mastering the thermal dissociation of the precursor, you transform a simple organic vapor into a precise tool for atomic-scale catalyst engineering.
Summary Table:
| Parameter | Specification/Process | Impact on Regeneration |
|---|---|---|
| Chlorine Source | 1,2-Dichloroethane (EDC) | Organic precursor for safe, controlled release |
| Control Mechanism | Gas flow ratio (EDC + Oxygen) | Dictates potential chlorine concentration |
| Operating Temp | 500 °C | Triggers thermal dissociation into active species |
| Active Species | Cl2 / HCl | Forms mobile Pt-O-Cl complexes for redispersion |
| Target Outcome | Atomic-scale redispersion | Reverses platinum aggregation for higher efficiency |
Optimize Your Catalyst Regeneration with KINTEK
Precise temperature control is the difference between failed dissociation and perfect catalyst redispersion. KINTEK provides high-performance reactor solutions designed to meet the rigorous 500°C demands of oxychlorination processes.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique lab or industrial needs. Whether you are managing complex in-situ chemical transformations or scaling up atomic-scale engineering, our equipment ensures the thermal stability your research requires.
Ready to enhance your lab's efficiency? Contact us today to discuss your custom furnace needs!
Visual Guide
References
- Lu Dong, Xinggui Zhou. Structure Robustness of Highly Dispersed Pt/Al2O3 Catalyst for Propane Dehydrogenation during Oxychlorination Regeneration Process. DOI: 10.3390/catal14010048
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does the carbon reductant ratio influence the selective reduction of ferronickel? Mastering Alloy Purity
- What are the core technical advantages of single-step microwave furnace sintering for SSBSN ceramics?
- How does Thermogravimetric Analysis (TGA/DTG) provide industrial guidance? Optimize Blast Furnace Dust Treatment
- What are advanced materials and composites? Unlock Superior Performance for Your Innovations
- What role do RTP or continuous sintering furnaces play in solar cell electrode formation? Optimize Your Firing Process
- What conditions are required for grafting norbornene functional groups onto S-glass fiber surfaces? Expert Protocol
- Why is a vibratory mill used for ultra-fine grinding when preparing magnesite samples for zeta potential tests?
- What mechanism causes the formation of micro-cracks in zinc clinker during microwave heating? Boost Leaching Efficiency



















