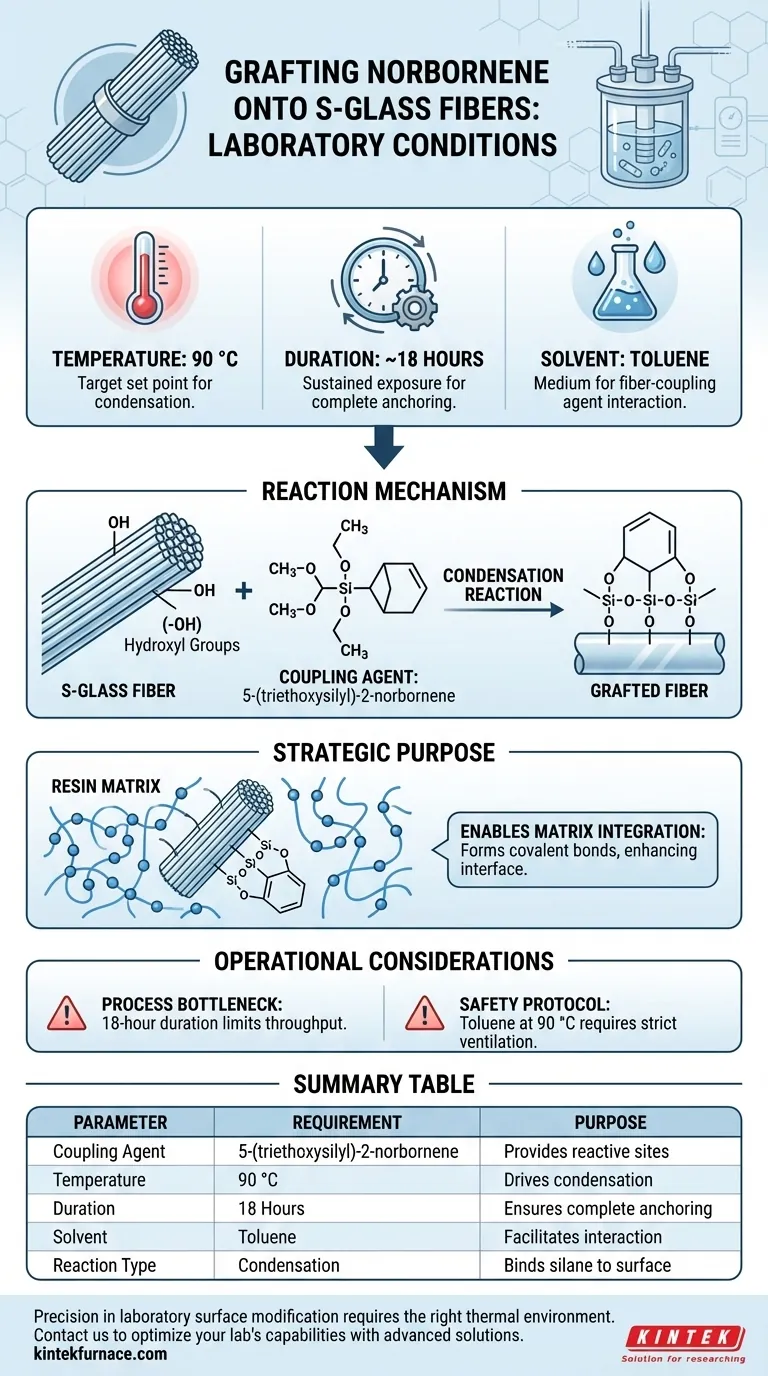
To successfully graft norbornene onto S-glass fibers, you must maintain a reaction environment at 90 °C for approximately 18 hours using toluene as the solvent. This specific setup facilitates a condensation reaction between the silane coupling agent, 5-(triethoxysilyl)-2-norbornene, and the hydroxyl groups naturally present on the fiber surface.
By strictly controlling temperature and duration in a toluene medium, this process chemically anchors reactive norbornene sites to the fiber. This modification transforms the fiber surface, allowing it to form covalent bonds with the resin matrix during subsequent polymerization.

The Chemistry of Surface Modification
The Primary Reactants
The process relies on the interaction between two specific components.
The first is the S-glass fiber surface, which provides the necessary hydroxyl (-OH) groups.
The second is the coupling agent, 5-(triethoxysilyl)-2-norbornene, which carries the functional group intended for grafting.
The Reaction Mechanism
The transformation is driven by a condensation reaction.
Under the specified laboratory conditions, the silane coupling agent reacts with the hydroxyl groups on the glass fiber.
This reaction chemically binds the silane to the glass, effectively "anchoring" the norbornene functionality to the surface.
Critical Processing Parameters
Thermal Requirements
The laboratory reaction equipment must be capable of maintaining a constant temperature.
The target set point is 90 °C. Consistency is vital to drive the condensation reaction to completion without degrading the reactants.
Duration of Exposure
This is not a rapid process; it requires sustained exposure to the reaction environment.
The standard duration for this protocol is approximately 18 hours.
Solvent Environment
The reaction medium is critical for facilitating the interaction between the solid fiber and the liquid coupling agent.
Toluene is the required solvent for this specific grafting procedure.
The Strategic Purpose
Creating Reactive Sites
The primary goal of this procedure is to alter the chemical nature of the fiber surface.
By grafting norbornene, you are installing specific chemical reaction sites onto an otherwise inert material.
Enabling Matrix Integration
This surface modification is a precursor to composite fabrication.
The anchored norbornene groups allow the fiber to participate directly in matrix polymerization.
This results in the formation of covalent bonds between the fiber and the resin, significantly enhancing the interface between the two materials.
Operational Considerations and Trade-offs
Process Efficiency vs. Quality
The 18-hour reaction time is a significant operational bottleneck.
While necessary for high-quality grafting under these specific conditions, it limits the throughput of fiber treatment in a laboratory setting.
Solvent Handling
The use of toluene at elevated temperatures (90 °C) requires strict safety protocols.
Laboratory equipment must be equipped with appropriate reflux or ventilation systems to manage solvent vapors over the extended reaction period.
Executing the Grafting Protocol
To ensure successful surface modification, align your laboratory setup with your specific experimental goals.
- If your primary focus is Process Fidelity: Strictly maintain the 90 °C temperature set point for the full 18-hour duration to ensure complete condensation.
- If your primary focus is Interface Engineering: Verify that your resin system is chemically compatible with norbornene groups to utilize the anchored sites for covalent bonding.
Success in this procedure relies on the precise combination of thermal energy, time, and solvent compatibility to permanently alter the fiber's chemical architecture.
Summary Table:
| Parameter | Requirement | Purpose |
|---|---|---|
| Coupling Agent | 5-(triethoxysilyl)-2-norbornene | Provides reactive norbornene sites |
| Temperature | 90 °C | Drives the condensation reaction |
| Duration | 18 Hours | Ensures complete chemical anchoring |
| Solvent | Toluene | Facilitates fiber-liquid interaction |
| Reaction Type | Condensation | Binds silane to surface hydroxyl groups |
Precision in laboratory surface modification starts with the right thermal environment. KINTEK provides the advanced high-temperature solutions necessary for complex chemical grafting and material synthesis. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of your lab research. Whether you are engineering interfaces or developing high-performance composites, our team is ready to provide the custom equipment your project requires. Contact us today to optimize your lab's capabilities!
Visual Guide
References
- Benjamin R. Kordes, Michael R. Buchmeiser. Ring‐Opening Metathesis Polymerization‐Derived Poly(dicyclopentadiene)/Fiber Composites Using Latent Pre‐Catalysts. DOI: 10.1002/mame.202300367
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Ultra High Vacuum CF Observation Window Flange with High Borosilicate Glass Sight Glass
- Ultra High Vacuum Observation Window KF Flange 304 Stainless Steel High Borosilicate Glass Sight Glass
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- Ultra High Vacuum CF Flange Stainless Steel Sapphire Glass Observation Sight Window
People Also Ask
- Why is the purity of oxide precursors critical for ZnO-doped CuO? Ensure High Photocatalytic Performance
- What are the methods of heat transfer in furnaces? Master Heat Control for Better Results
- Why is a pre-heated oxygen blowing system essential for chalcopyrite ignition? Ensure Precise Flash Smelting Simulation
- What is the specific function of laboratory electric heating devices in solid-state hydrogen storage? Optimize Thermal Management
- What are the advantages of a Vacuum Drying Oven for NiCo2O4 nanosheet composites? Protect Your Nanostructural Integrity
- What role does a high-temperature annealing furnace play in the preparation of AAO substrates? Enhance Pore Regularity
- What is the purpose of using a vacuum drying oven for mineral powders? Optimize Polymer Bonding and Density
- How is a CCD camera used for iron ore pellet deformation? Master Non-Contact Strain Measurement at High Temperatures



