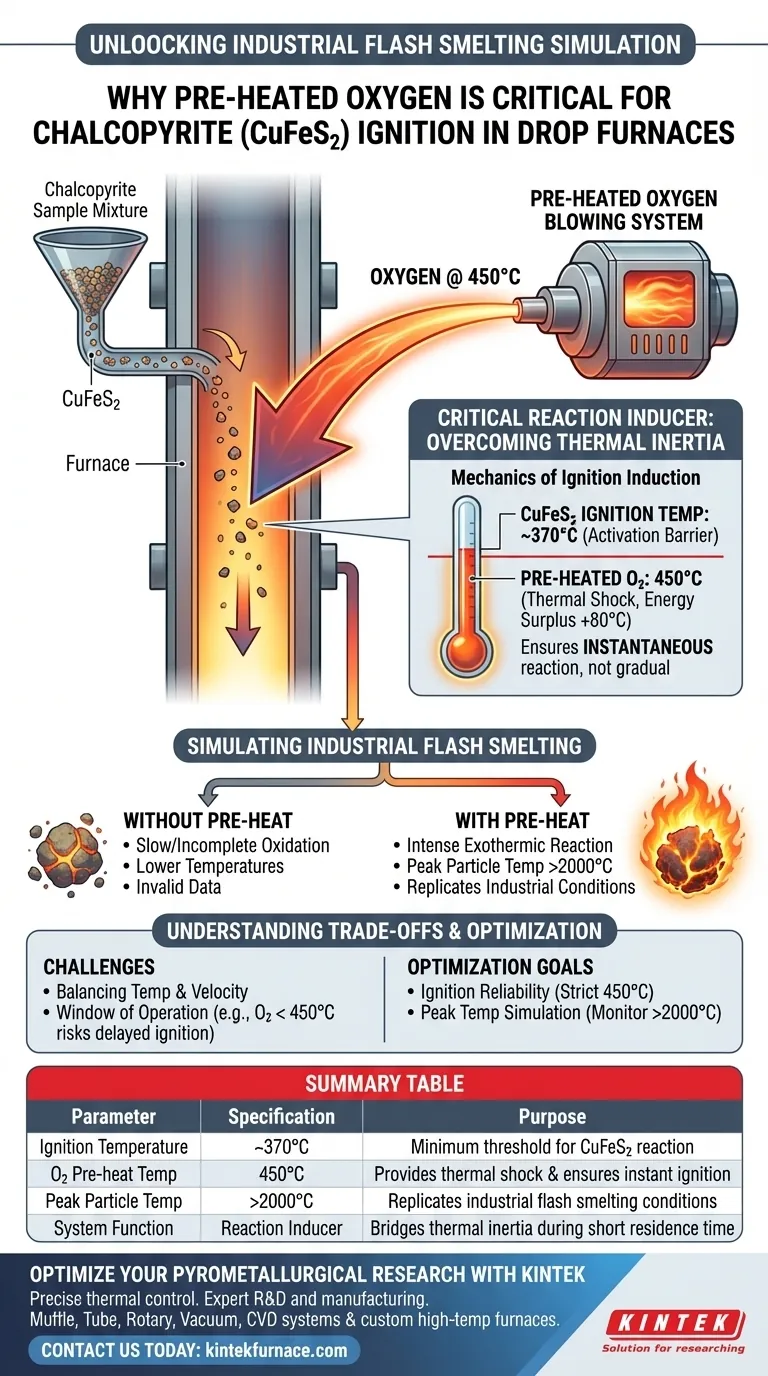
The pre-heated oxygen blowing system functions as a critical reaction inducer, essential for overcoming the thermal inertia of chalcopyrite (CuFeS2) in a drop furnace setting. By delivering oxygen at 450°C directly onto the sample mixture, the system ensures the environment exceeds the mineral's ignition temperature of approximately 370°C. This mechanism is required to trigger the instantaneous thermal decomposition and oxidation necessary to simulate industrial flash smelting.
In drop furnace experiments, ambient heat alone is often insufficient to trigger rapid ignition during the short residence time of a falling particle. The pre-heated oxygen blast bridges this gap, forcing immediate ignition and driving particle temperatures to over 2000°C to replicate the intense exothermic conditions of a flash furnace.

The Mechanics of Ignition Induction
Overcoming the Activation Barrier
Chalcopyrite requires a specific thermal threshold to begin reacting. The mineral has an ignition temperature of approximately 370°C.
Below this temperature, the sulfide structure remains relatively stable. To ensure a reaction occurs within the limited timeframe of a drop test, the environment must immediately exceed this threshold.
The Role of Thermal Shock
The blowing system does not merely warm the sample; it delivers a thermal shock. By pre-heating the oxygen to 450°C, the system provides an energy surplus of roughly 80°C above the ignition point.
This surplus guarantees that when the oxygen stream contacts the sample mixture, the reaction is not gradual but instantaneous. This mimics the aggressive reaction kinetics found in large-scale processing.
Simulating Industrial Flash Smelting
Replicating Exothermic Intensity
Industrial flash furnaces rely on the heat generated by the burning ore to sustain the process. In a laboratory drop furnace, the scale is too small to naturally generate this "flash" effect without assistance.
The pre-heated oxygen initiates the intense oxidation required to liberate sulfur and iron. Once triggered, this exothermic reaction becomes self-sustaining during the particle's descent.
Achieving Peak Temperatures
The ultimate goal of the experiment is to study the particle under extreme heat. The initial kick from the pre-heated oxygen drives the particle temperature upward rapidly.
According to experimental data, this method ensures particles reach peak temperatures exceeding 2000°C. Without the pre-heated induction, the particles might oxidize slowly or incompletely, failing to generate the high temperatures characteristic of real-world smelting.
Understanding the Trade-offs
Balancing Temperature and Velocity
While pre-heating is essential, the velocity of the blowing system introduces a variable that must be managed. A high-velocity stream ensures good oxidant contact but can alter the aerodynamic trajectory of the falling particles.
The Window of Operation
The system relies on a specific temperature differential. If the oxygen temperature drops below the 450°C target, it risks falling too close to the 370°C ignition threshold.
This reduced margin for error can lead to delayed ignition. Delayed ignition results in the particle reaching the bottom of the furnace before fully reacting, yielding invalid data.
Optimizing Your Experimental Setup
To ensure valid data collection in chalcopyrite drop tests, align your parameters with your specific research goals:
- If your primary focus is Ignition Reliability: Maintain the oxygen pre-heat temperature strictly at 450°C to ensure it stays well above the 370°C activation threshold.
- If your primary focus is Peak Temperature Simulation: Monitor the reaction zone to confirm that the initial oxidation kick is successfully driving particle temperatures beyond 2000°C.
Control over the pre-heated oxygen stream is the single most important factor in bridging the gap between laboratory scale experiments and industrial reality.
Summary Table:
| Parameter | Specification | Purpose |
|---|---|---|
| Ignition Temperature | ~370°C | Minimum threshold for CuFeS2 reaction |
| O2 Pre-heat Temp | 450°C | Provides thermal shock & ensures instant ignition |
| Peak Particle Temp | >2000°C | Replicates industrial flash smelting conditions |
| System Function | Reaction Inducer | Bridges thermal inertia during short residence time |
Optimize Your Pyrometallurgical Research with KINTEK
Precise thermal control is the difference between valid data and failed experiments. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab high-temp furnaces tailored for complex minerals like chalcopyrite.
Whether you need to simulate flash smelting or achieve extreme temperature gradients, our engineering team provides the reliability you need. Contact us today to discuss your custom furnace requirements and see how our advanced heating solutions can drive your lab's success.
Visual Guide
References
- Motoo KAWASAKI, Hiromichi Takebe. Evaluation of Ignition and Combustion Reactions of CuFeS<sub>2</sub> and Silica Stone Less Than 100 ms in a Drop Furnace. DOI: 10.2473/journalofmmij.mmij-2024-010
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- How do heat treatment furnaces function? Master Thermal Control and Atmosphere for Superior Material Properties
- What is the role of high-purity helium in electromagnetic levitation? Key for Rapid Thermal Regulation
- What is the purpose of magnetron sputtering in N-I-P CsPbBr3 detectors? Optimize Charge Transport & Stability
- How does the elimination of double oxide films improve T7 over-aging? Unlock Superior Ductility in Aluminum Alloys
- How does the orientation of glass within a tempering furnace affect quality? Optimize Optical and Physical Properties
- What factors should be considered when selecting a furnace based on material properties? Ensure Optimal Heat Treatment
- Why is chemical or mechanical cleaning required after high-temperature diffusion coating? Ensure Precision & Quality
- What protective roles does argon gas play in SiC sintering? Essential Insights for High-Purity Ceramics



















