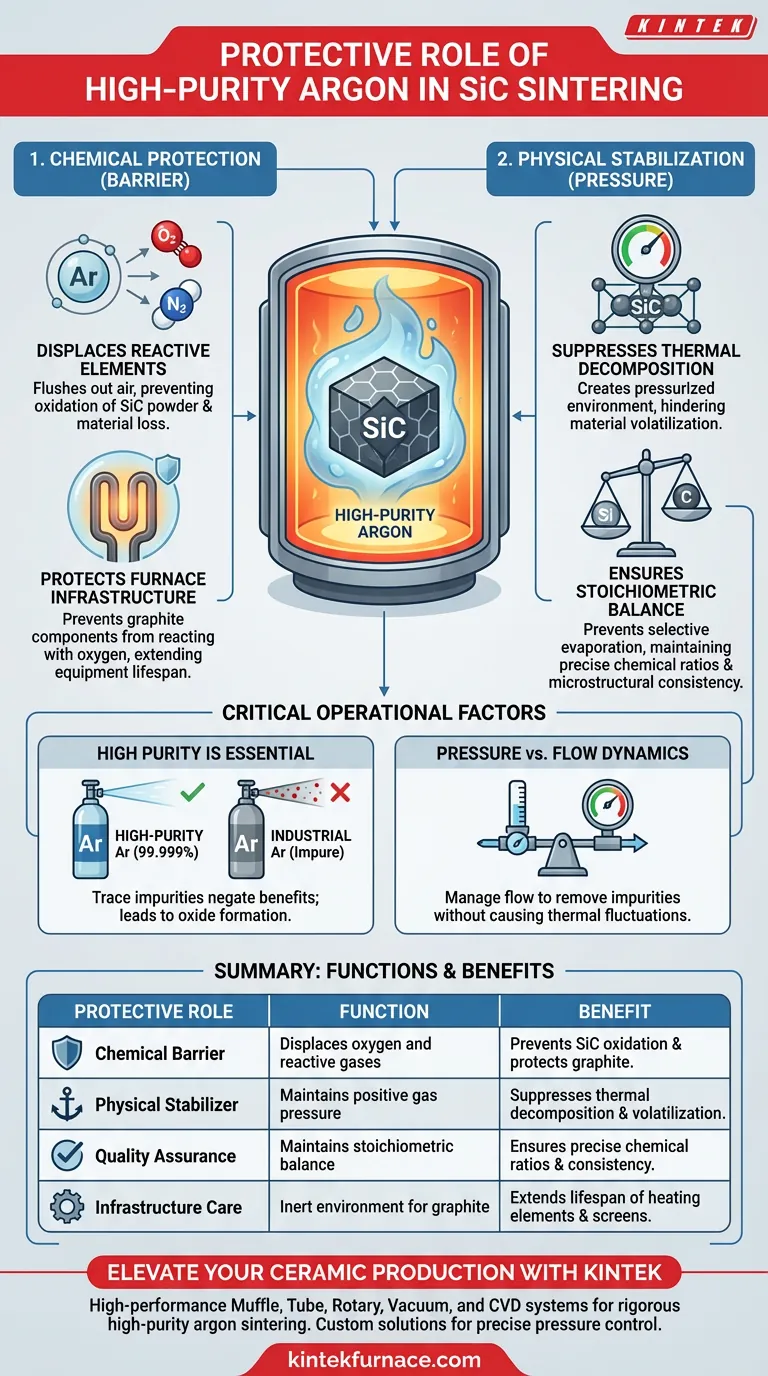
The continuous supply of high-purity argon gas is essential for preserving the chemical and structural integrity of Silicon Carbide (SiC). During high-temperature sintering, this inert gas performs two critical functions: it displaces oxygen to prevent the oxidation of both the SiC powder and graphite furnace components, and it creates a pressurized environment that suppresses the thermal decomposition of the material. Without this protective atmosphere, the ceramic would suffer from compositional loss and structural degradation.
High-purity argon acts as both a chemical barrier against oxidation and a physical stabilizer against volatilization. By maintaining a stable positive pressure, it ensures the final Silicon Carbide ceramic retains its precise stoichiometric balance and intended microstructure.

The Role of Argon in Chemical Protection
Displacing Reactive Elements
The primary threat during sintering is the presence of residual oxygen in the furnace chamber.
High-purity argon serves as a displacement medium, flushing out air that would otherwise react with the material.
Without this displacement, Silicon Carbide powder would oxidize, leading to significant material loss and surface defects on the final product.
Protecting Furnace Infrastructure
The protection extends beyond the ceramic product to the furnace itself.
Most high-temperature sintering furnaces utilize graphite components, such as heating elements and insulation screens.
Argon prevents these carbon-based components from reacting with oxygen and burning away, thereby extending the lifespan of your critical equipment.
The Role of Argon in Physical Stabilization
Suppressing Thermal Decomposition
At the extreme temperatures required for sintering, Silicon Carbide is prone to thermal decomposition.
This process involves the material breaking down and components volatilizing (turning into gas) rather than densifying.
A stable, continuous supply of argon creates a protective gas pressure that physically suppresses this volatilization tendency.
Ensuring Stoichiometric Balance
The quality of a technical ceramic is defined by its chemical ratio, or stoichiometry.
By preventing the selective evaporation of silicon or carbon species, argon ensures the chemical balance remains constant throughout the process.
This stability is what guarantees the microstructural consistency required for high-performance ceramic applications.
Understanding the Operational Trade-offs
The Necessity of High Purity
The "inert" nature of argon is only effective if the gas is strictly high-purity.
Using industrial-grade argon with trace impurities (such as moisture or oxygen) will negate the protective benefits.
Even a small amount of contamination can lead to the formation of unwanted oxide layers, compromising the material properties.
Pressure vs. Flow Dynamics
While pressure suppresses decomposition, the flow rate must be carefully managed.
Stagnant gas may not effectively remove evolving impurities from the binder burnout phase.
Conversely, excessive flow can cause thermal fluctuations within the hot zone, potentially affecting the uniformity of the sintering temperature.
Making the Right Choice for Your Goal
To maximize the quality of your Silicon Carbide sintering, align your gas management strategy with your specific objectives:
- If your primary focus is microstructural density: Prioritize precise pressure control to suppress volatilization and maintain strict stoichiometric balance.
- If your primary focus is equipment longevity: Ensure a consistent, uninterrupted flow to keep oxygen levels near zero, protecting graphite heating elements from degradation.
Mastering the atmosphere is just as critical as mastering the temperature profile for successful SiC ceramics.
Summary Table:
| Protective Role | Function | Benefit |
|---|---|---|
| Chemical Barrier | Displaces oxygen and reactive gases | Prevents SiC oxidation and protects graphite furnace components |
| Physical Stabilizer | Maintains positive gas pressure | Suppresses thermal decomposition and material volatilization |
| Quality Assurance | Maintains stoichiometric balance | Ensures precise chemical ratios and microstructural consistency |
| Infrastructure Care | Inert environment for graphite | Extends the lifespan of heating elements and insulation screens |
Elevate Your Advanced Ceramic Production with KINTEK
Achieving the perfect stoichiometric balance in Silicon Carbide requires more than just heat; it requires a perfectly controlled atmosphere. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to handle the rigorous demands of high-purity argon sintering.
Whether you need precise pressure control to suppress volatilization or customizable high-temp furnaces to protect your unique materials, our technical team is ready to assist. Contact us today to discover how our customizable lab solutions can optimize your sintering process and enhance your material's structural integrity.
Visual Guide
References
- Peroxymonosulfate Activation by Sludge-Derived Biochar via One-Step Pyrolysis: Pollutant Degradation Performance and Mechanism. DOI: 10.3390/w17172588
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
People Also Ask
- What is the role of carbonaceous reducing agents in copper slag treatment? Maximize Metal Recovery with Expert Insights
- What type of furnace was chosen for annealing silicon-based materials and what were the key requirements? Discover the Ideal Solution for Precise Heat Treatment
- What are the material and structural requirements for heating walls? Optimize Your Externally Heated Retorting Furnace
- How are impurity levels controlled during tantalum powder synthesis? Master High-Purity Magnesiothermic Reduction
- What is the necessity of a laboratory vacuum drying oven for photocatalytic powders? Protect Your Material Integrity
- What is the function of ball milling in Li-NASICON synthesis? Optimize Your Solid Electrolyte Performance
- What is the purpose of sintering in materials science? Transform Powders into Dense, High-Strength Components
- Why is high-purity argon necessary for PVC dechlorination? Ensure Precise Reaction Control & Safety



















