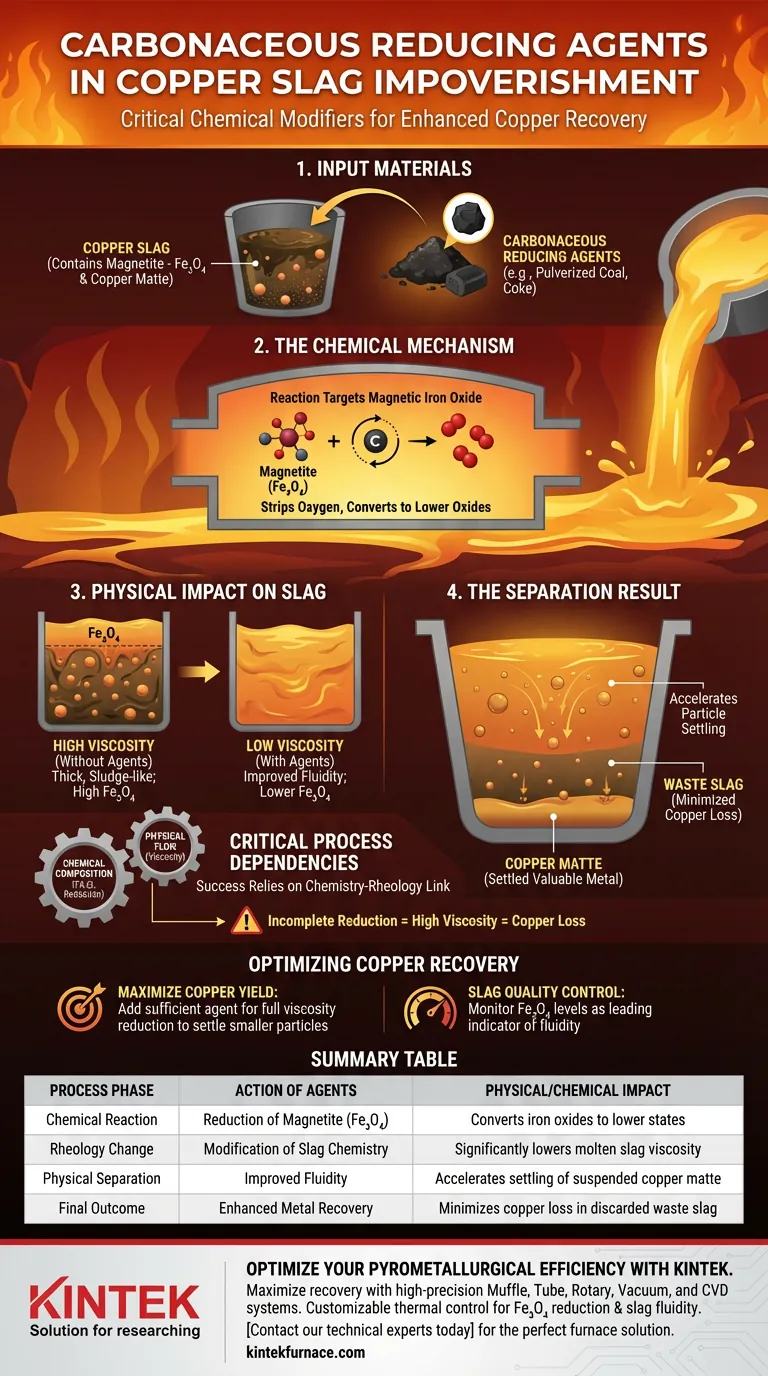
Carbonaceous reducing agents act as critical chemical modifiers in the pyrometallurgical impoverishment of copper slag. By introducing materials such as pulverized coal or coke into the molten mixture, operators trigger a specific chemical reduction that fundamentally alters the physical properties of the slag to enable copper recovery.
The primary function of these agents is to reduce magnetic iron oxide ($Fe_3O_4$) into lower oxides. This chemical transformation lowers the viscosity of the molten slag, facilitating the physical settling of copper matte particles and preventing valuable metal from being lost in the waste.
The Chemical Mechanism
Targeting Magnetic Iron Oxide
Copper slag naturally contains significant amounts of magnetic iron oxide, known as magnetite ($Fe_3O_4$).
This compound is the primary target of the impoverishment process.
The Reduction Reaction
When carbonaceous agents (like coal or coke) are added to the melt, they react with the magnetite.
This reaction strips oxygen from the magnetite, converting the $Fe_3O_4$ into lower oxides.
The Physical Impact on Slag
Lowering Viscosity
The presence of high levels of magnetite tends to make molten slag thick and sludge-like.
By chemically reducing the magnetite to lower oxides, the carbonaceous agents significantly lower the viscosity of the fluid.
Improving Fluidity
The reduction process directly results in improved fluidity.
A more fluid slag creates an environment where suspended particles encounter less resistance to movement.
The Separation Result
Accelerating Particle Settling
Valuable copper exists within the slag as suspended copper matte particles.
The increased fluidity accelerates the settling of these heavier particles toward the bottom of the vessel.
Reducing Copper Loss
Efficient settling allows for a distinct separation between the valuable matte and the waste slag.
This separation ensures that the copper content remaining in the discarded slag is significantly minimized.
Critical Process Dependencies
The Link Between Chemistry and Rheology
The success of this process relies entirely on the relationship between chemical composition and physical flow.
If the magnetic iron oxide is not sufficiently reduced, the slag remains too viscous.
The Consequence of Incomplete Reduction
High viscosity acts as a physical barrier to separation.
Without the addition of reducing agents, copper matte particles remain trapped in the suspension and are lost in the final waste stream.
Optimizing Copper Recovery
To effectively manage the impoverishment process, align your approach with the following operational goals:
- If your primary focus is maximizing copper yield: Ensure sufficient carbonaceous agent is added to fully lower the viscosity, allowing even smaller matte particles to settle out.
- If your primary focus is slag quality control: Monitor the levels of magnetic iron oxide ($Fe_3O_4$), as its reduction is the leading indicator of improved fluidity and separation efficiency.
Mastering the viscosity of the slag through chemical reduction is the single most effective lever for minimizing copper loss.
Summary Table:
| Process Phase | Action of Carbonaceous Agents | Physical/Chemical Impact |
|---|---|---|
| Chemical Reaction | Reduction of Magnetite ($Fe_3O_4$) | Converts iron oxides to lower states |
| Rheology Change | Modification of Slag Chemistry | Significantly lowers molten slag viscosity |
| Physical Separation | Improved Fluidity | Accelerates settling of suspended copper matte |
| Final Outcome | Enhanced Metal Recovery | Minimizes copper loss in discarded waste slag |
Optimize Your Pyrometallurgical Efficiency with KINTEK
Maximize your metal recovery and refine your copper slag treatment with high-precision equipment. Backed by expert R&D and manufacturing, KINTEK offers high-temperature Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific metallurgical needs.
Whether you are targeting $Fe_3O_4$ reduction or improving slag fluidity, our lab furnaces provide the precise thermal control required for successful impoverishment processes.
Ready to enhance your lab's productivity? Contact our technical experts today to find the perfect furnace solution for your application.
Visual Guide
References
- Jiaxing Liu, Baisui Han. The Utilization of the Copper Smelting Slag: A Critical Review. DOI: 10.3390/min15090926
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the primary function of drying bovine horn biomass for PVC biocomposites? Optimize Material Strength
- Why is vacuum freeze-drying necessary for FeNC/MXene catalysts? Preserving 2D Architecture for Peak Performance
- Why are precision stirring and drying equipment necessary for photocatalytic materials? Master Microstructure Control
- What role does a constant temperature water bath play in simulated hot-rolling oxidation? Master Precision Humidity
- Why is a high-purity argon flow control system essential? Ensure Precision in Metallurgy Simulations
- What are the specific functions of a flowing 5% H2/Ar gas mixture? Master Thermal Reduction of Nanoparticles
- Why is rapid water quenching necessary for Ce2(Fe, Co)17 alloys? Unlock Peak Magnetocaloric Performance
- How does low-temperature volatilization equipment function? Efficient Electrolyte Removal for Battery Recycling














