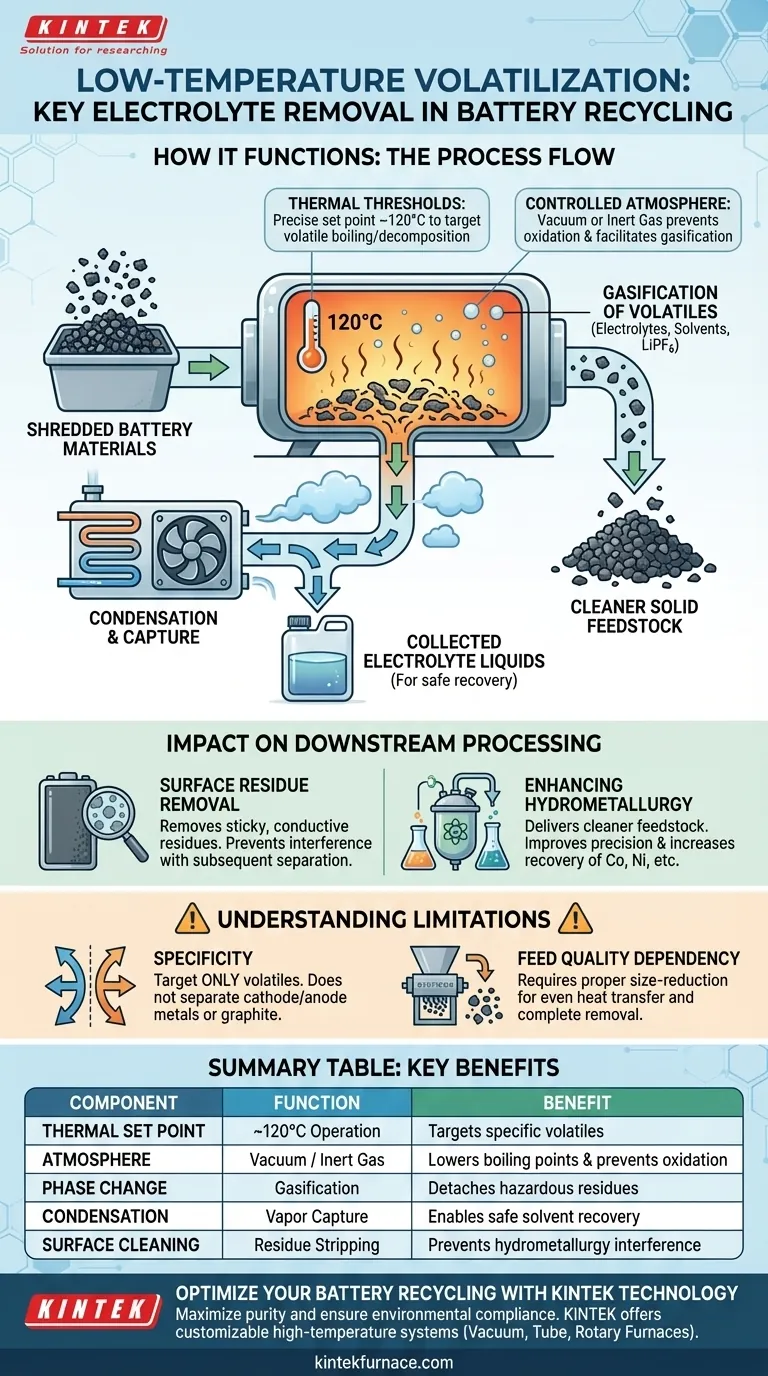
Low-temperature volatilization equipment functions by heating shredded battery materials to approximately 120°C within a strictly controlled environment, typically utilizing a vacuum or inert gas atmosphere. This thermal treatment triggers a phase change in volatile components—specifically organic solvents and lithium hexafluorophosphate—converting them from liquid or solid residues into gas. By isolating these vapors and subsequently condensing them, the system effectively strips the electrolyte from the solid active materials without requiring the extreme heat of pyrometallurgy.
Low-temperature volatilization serves as a critical purification stage that decouples hazardous electrolyte recovery from metal recycling. By removing these residues early, the process prevents contamination and significantly enhances the efficiency of subsequent hydrometallurgical operations.

The Mechanics of Separation
Thermal Thresholds
The equipment operates at a precise thermal set point of approximately 120°C. This temperature is carefully selected to target the boiling points and decomposition temperatures of specific volatile compounds found in battery electrolytes.
Controlled Atmosphere
To facilitate efficient gasification and prevent unwanted combustion, the process occurs under vacuum or inert gas conditions. A vacuum environment lowers the boiling point of solvents, allowing them to vaporize with less energy, while inert gas prevents oxidation of the exposed metal components.
Material Recovery Process
Gasification of Volatiles
As the shredded material reaches the target temperature, the electrolyte components—including lithium hexafluorophosphate and various organic solvents—transition into a gaseous state. This effectively detaches them from the surface of the cathode and anode materials.
Condensation and Capture
The gasified electrolytes are drawn away from the solid material stream. These vapors are then routed through a cooling system where they condense back into liquids for safe collection and potential recovery.
Impact on Downstream Processing
Surface Residue Removal
The primary function of this equipment is to clean the surface of active materials. Removing the sticky, conductive electrolyte residues prevents them from interfering with mechanical separation or chemical leaching processes later in the recycling line.
Enhancing Hydrometallurgy
By delivering cleaner feedstock to the hydrometallurgical stage, the equipment improves overall process efficiency. The absence of interfering organic solvents allows for more precise chemical reactions and higher recovery rates of valuable metals like cobalt and nickel.
Understanding the Limitations
Specificity of Removal
This equipment is designed strictly for volatile components. It does not separate the cathode metals from the anode graphite or current collectors; it only prepares the mixture for those subsequent separation steps.
Dependency on Feed Quality
The efficiency of the volatilization depends on the material being properly shredded first. If the battery materials are not adequately size-reduced, heat transfer may be uneven, leading to incomplete removal of the electrolyte deep within the material mass.
Optimizing the Recycling Workflow
To determine where this equipment fits into your process, consider your specific recovery targets.
- If your primary focus is safety and environmental compliance: This step is essential for capturing hazardous lithium hexafluorophosphate and solvents before they can be released as emissions or create safety hazards in downstream acid leaching.
- If your primary focus is hydrometallurgical yield: Utilizing this equipment maximizes the purity of your black mass feedstock, preventing organic contamination from reducing the efficiency of your chemical recovery circuits.
Effective electrolyte removal acts as the gateway to high-purity metal recovery in modern battery recycling.
Summary Table:
| Process Component | Functional Role | Key Benefit |
|---|---|---|
| Thermal Set Point | Operates at ~120°C | Targets specific boiling points of volatiles |
| Controlled Atmosphere | Vacuum or Inert Gas | Lowers boiling points & prevents oxidation |
| Phase Change | Gasification of Electrolytes | Detaches hazardous residues from active solids |
| Condensation | Vapor Capture & Cooling | Enables safe collection & recovery of solvents |
| Surface Cleaning | Residue Stripping | Prevents interference in hydrometallurgy |
Optimize Your Battery Recycling with KINTEK Technology
Maximize the purity of your black mass and ensure environmental compliance with KINTEK’s advanced thermal solutions. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of laboratory high-temperature systems—including Vacuum, Tube, and Rotary furnaces—all customizable to meet the unique demands of your electrolyte recovery and material purification processes.
Ready to enhance your hydrometallurgical yields? Contact us today to find the perfect solution for your lab!
Visual Guide
References
- Muammer Kaya, Hossein Delavandani. State-of-the-Art Lithium-Ion Battery Pretreatment Methods for the Recovery of Critical Metals. DOI: 10.3390/min15050546
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the design logic behind the double-layer reactor structure used in the ITSP process? Optimize Your Fuel Quality
- What are the technical advantages of using a flux-coated filler metal with 20% silver? Optimize Cost & Joint Integrity
- What process problems are addressed by using a walking-beam furnace model? Solve Clad Plate Thermal Stress Challenges
- Why is the melt-diffusion technique employed at 155 °C for sulfur cathode composites? Master Precise Infiltration
- Why is precise temperature control critical for drying carbon nanotube films? Achieve Perfect 80°C Thermal Stability
- What are the advantages of zirconia crowns? Achieve Durable, Aesthetic, and Biocompatible Dental Restorations
- What is the function of a Laboratory Forced Air Drying Oven in fruit waste pretreatment? Ensure Superior Carbon Yields
- How does a high-temperature laboratory furnace contribute to the formation of high-quality CsV3Sb5 single crystals?



















