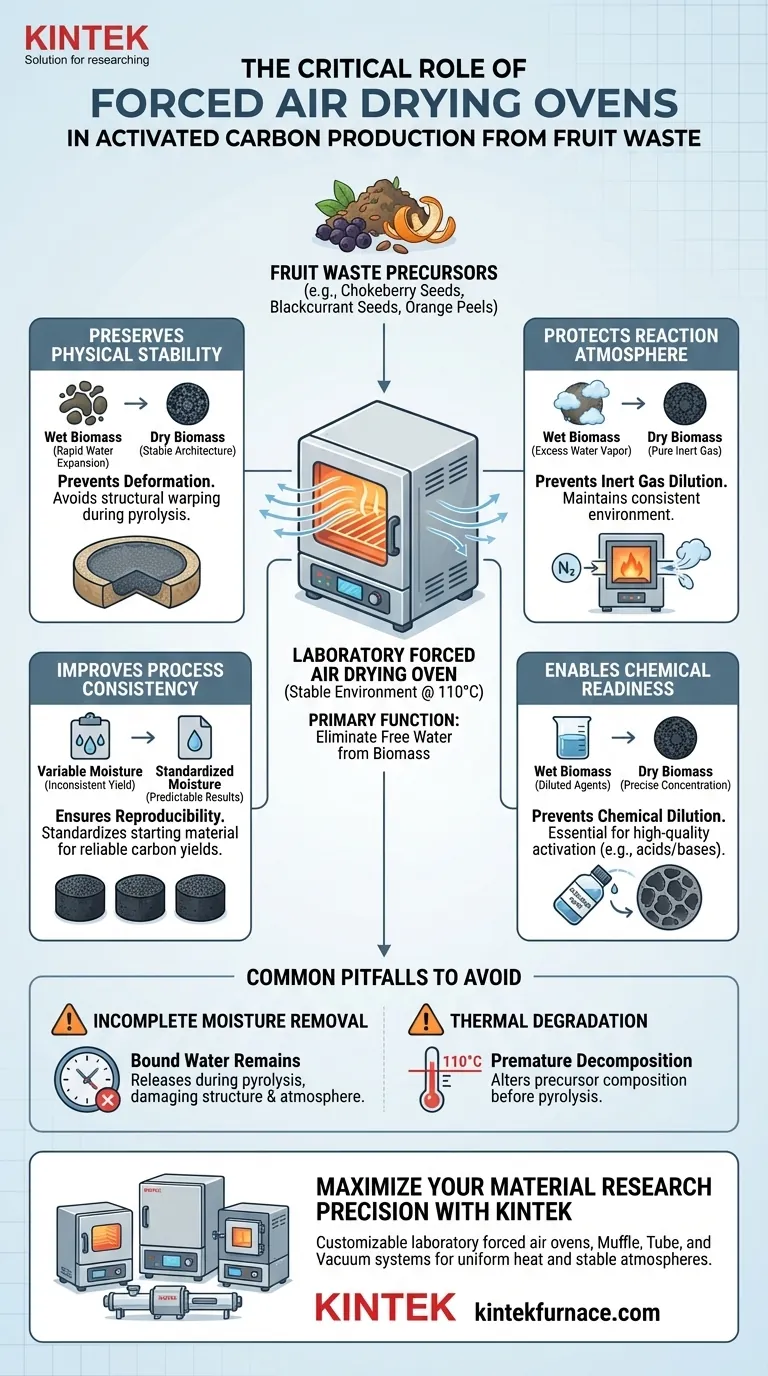
The primary function of a Laboratory Forced Air Drying Oven in the pretreatment of fruit waste is to eliminate free water from biomass materials by maintaining a stable environment, typically at 110 °C. This dehydration step is a prerequisite for stabilizing precursors such as chokeberry seeds, blackcurrant seeds, and orange peels before they undergo high-temperature processing.
By thoroughly removing moisture, the drying oven prevents physical deformation of the material and maintains the integrity of the inert atmosphere inside the reaction furnace. This ensures experimental reproducibility and prevents water vapor from interfering with critical chemical reactions.
Preserving Physical and Chemical Integrity
The drying process is not merely about weight reduction; it is about preparing the molecular structure of the fruit waste for carbonization.
Preventing Physical Deformation
When biomass containing free water is subjected to the intense heat of pyrolysis, the water expands rapidly.
This rapid expansion can cause irregular physical deformation of the precursor structure. By using a forced air oven to remove this water gently at 110 °C, you ensure the physical architecture of the seeds or peels remains stable during the transition to activated carbon.
Protecting the Reaction Atmosphere
Pyrolysis relies on a strictly controlled inert atmosphere to prevent combustion and encourage carbon formation.
If wet biomass enters the furnace, it releases significant amounts of excess water vapor. This vapor dilutes the inert gas (such as nitrogen) within the reaction chamber, creating an inconsistent environment that alters the chemical outcome of the experiment.
Ensuring Experimental Reproducibility
Scientific rigor demands that every batch of activated carbon performs predictably.
Variable moisture content in fruit waste leads to variable carbon yields and surface properties. A forced air drying oven standardizes the starting material, thereby significantly improving the reproducibility of your experimental results.
The Role of Drying in Chemical Activation
While the primary reference focuses on physical and atmospheric stability, thorough drying is also critical for subsequent chemical steps.
Preventing Chemical Dilution
If you plan to use chemical activation agents, such as acids or bases, moisture control is vital.
Residual water in the biomass can block the pores or dilute chemical impregnating agents. Ensuring the material is completely dry prevents moisture from interfering with the precise concentration of activation agents, a principle essential for high-quality carbonization.
Common Pitfalls to Avoid
Using a drying oven seems straightforward, but specific errors can compromise the entire activated carbon production line.
Incomplete Moisture Removal
Setting the duration too short or the temperature too low leaves "bound" water within the cellular structure.
This residual moisture will eventually release during pyrolysis, leading to the exact atmospheric dilution and structural damage you are trying to avoid.
Thermal Degradation
While drying is necessary, excessive heat during this stage is detrimental.
Raising the temperature significantly above 110 °C may start premature decomposition of the organic components in the fruit waste. This alters the precursor's composition before it ever reaches the pyrolysis reactor.
Making the Right Choice for Your Goal
To maximize the quality of your activated carbon, align your drying protocol with your specific experimental objectives.
- If your primary focus is Structural Integrity: Ensure the oven temperature is strictly maintained at 110 °C to prevent rapid expansion and warping of the precursor.
- If your primary focus is Process Consistency: Utilize the forced air mechanism to guarantee uniform heat distribution, ensuring that water vapor does not dilute the inert atmosphere of the furnace.
- If your primary focus is Chemical Activation: Verify the complete removal of free water to prevent the dilution of impregnating agents during the activation stage.
A disciplined drying process is the invisible foundation upon which high-performance activated carbon is built.
Summary Table:
| Function Category | Key Benefit | Technical Impact |
|---|---|---|
| Physical Stability | Prevents Deformation | Eliminates rapid water expansion and warping during pyrolysis. |
| Atmospheric Control | Protects Inert Gas | Prevents water vapor from diluting nitrogen or other inert atmospheres. |
| Process Consistency | Improves Reproducibility | Standardizes moisture levels to ensure predictable carbon yields. |
| Chemical Readiness | Prevents Dilution | Ensures activation agents (acids/bases) maintain precise concentrations. |
| Thermal Safety | Prevents Degradation | Controlled 110°C drying avoids premature decomposition of organics. |
Maximize Your Material Research Precision
Unreliable drying protocols can compromise your entire activated carbon production line. Backed by expert R&D and manufacturing, KINTEK offers high-performance laboratory forced air ovens, Muffle, Tube, and Vacuum systems specifically designed to meet the rigorous demands of material science.
Whether you are processing fruit waste precursors or advanced chemical compounds, our customizable solutions ensure uniform heat distribution and stable atmospheres for every experiment. Don’t let moisture variability ruin your results—Contact KINTEK today to optimize your lab's thermal processing workflow!
Visual Guide
References
- Sylwia Kukowska, Katarzyna Szewczuk‐Karpisz. New fruit waste-derived activated carbons of high adsorption performance towards metal, metalloid, and polymer species in multicomponent systems. DOI: 10.1038/s41598-025-85409-0
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is shrinkage in the context of high-temperature materials? Master Dimensional Control for Stronger Parts
- What are the advantages of SLRP compared to traditional high-temperature furnaces? Revolutionizing UHTC Coatings
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control
- What is the mechanism of solution treatment on Cu-Cr-Zr-La alloys? Master the Thermal Cycle for High-Strength Alloys
- What are the technical advantages of vacuum drying ovens for CeO2 separators? Protect Nanostructures & Boost Stability
- What is Joule Heating and how does it relate to induction heating? Master the Physics of Contactless Heating
- What are some drawbacks of electric heating methods? High Costs and Grid Dependence Explained
- Why is the melt-diffusion technique employed at 155 °C for sulfur cathode composites? Master Precise Infiltration



















