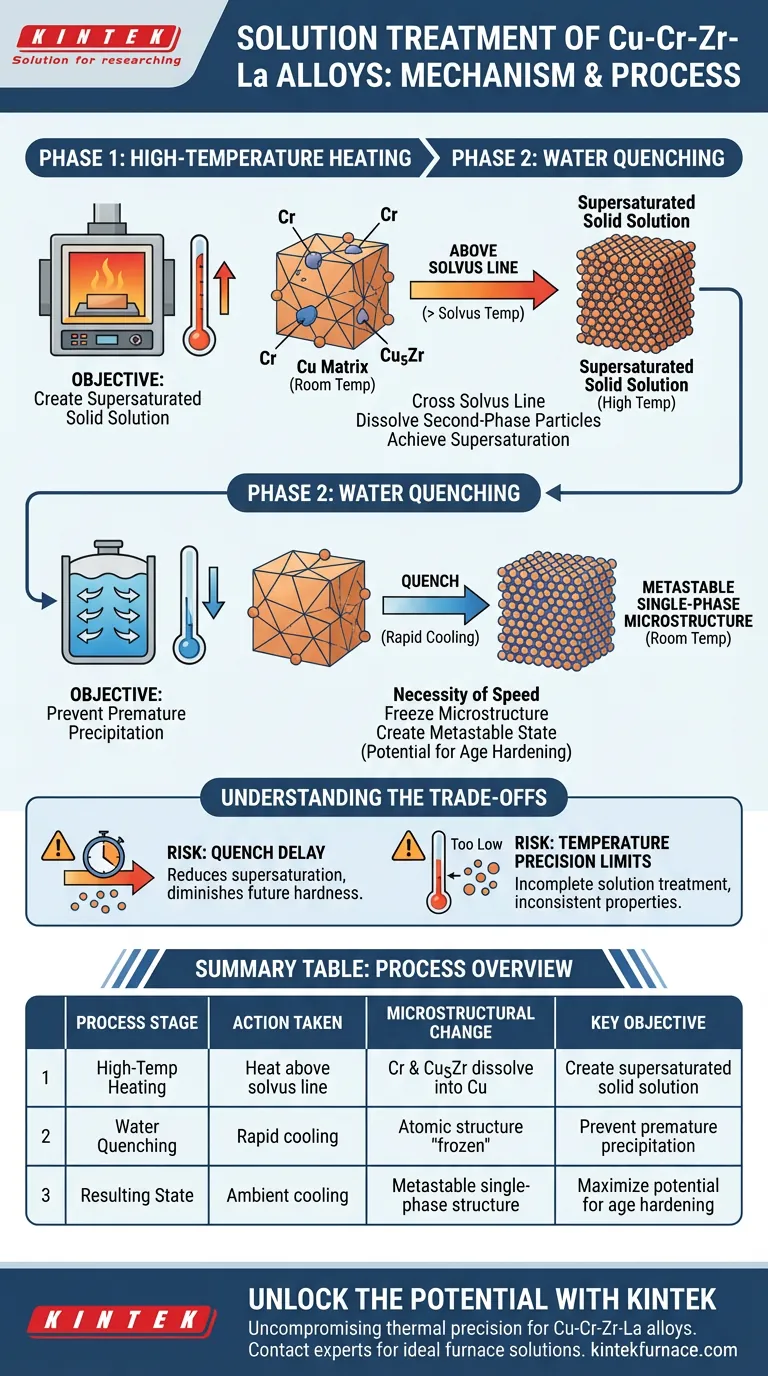
The solution treatment mechanism relies on a precise two-stage thermal cycle designed to manipulate the alloy's atomic structure. First, a high-temperature furnace heats the Cu-Cr-Zr-La alloy above its solvus line to dissolve second-phase particles like Chromium (Cr) and Cu5Zr directly into the copper matrix. This is immediately followed by water quenching, which utilizes an extremely high cooling rate to trap these elements in a supersaturated state before they can precipitate out.
The ultimate goal of this process is to create a metastable single-phase microstructure. By dissolving precipitates and "freezing" the atomic structure instantaneously, you establish the necessary thermodynamic driving force for subsequent age hardening.

The Physics of High-Temperature Dissolution
Crossing the Solvus Line
The process begins by raising the temperature of the alloy within a furnace. The target temperature must exceed the "solvus line," a specific thermodynamic threshold for the material. Crossing this threshold is the prerequisite for changing the phase stability of the alloy's constituents.
Dissolving Second-Phase Particles
Once the temperature is sufficiently high, distinct particles within the alloy begin to break down. Specifically, Chromium (Cr) and Cu5Zr particles lose their separate identity. They dissolve completely, diffusing into the surrounding copper lattice.
Achieving Supersaturation
The result of this heating phase is the formation of a solid solution. At this elevated temperature, the copper matrix holds more solute atoms (Cr and Zr) than it naturally could at room temperature. This state is known as a supersaturated solid solution.
The Critical Role of Water Quenching
The Necessity of Speed
Once the solid solution is formed, the alloy is subjected to immediate water quenching. The speed of this transition is the single most critical factor in the process. The cooling rate must be extremely high to prevent the dissolved atoms from moving back into their equilibrium states.
Freezing the Microstructure
The rapid drop in temperature effectively "freezes" the atomic structure. It prevents the diffusion that would normally allow the Cr and Cu5Zr to re-form as separate particles.
Creating a Metastable State
The final product of the quenching phase is a metastable, single-phase microstructure at room temperature. While this state is not chemically stable in the long term, it is locked in place kinetically. This trapped energy provides the potential required for the alloy to be strengthened during later aging processes.
Understanding the Trade-offs
The Risk of Quench Delay
The transition from the furnace to the water quench must be immediate. Any delay allows the temperature to drop slowly, which permits precipitates to form prematurely. This reduces the supersaturation level and diminishes the effectiveness of future hardening.
Temperature Precision Limits
While high heat is required, the temperature must be controlled relative to the solvus line. If the temperature is too low, the Cr and Cu5Zr particles will not fully dissolve. This results in an incomplete solution treatment and inconsistent mechanical properties in the final product.
Optimizing the Treatment Process
To maximize the performance of Cu-Cr-Zr-La alloys, you must align the process parameters with your specific metallurgical objectives.
- If your primary focus is maximizing future hardness: Ensure the quenching rate is as rapid as possible to lock in the highest degree of supersaturation.
- If your primary focus is microstructural homogeneity: Verify that the furnace temperature remains consistently above the solvus line long enough to ensure total particle dissolution.
Precise control of this thermal cycle is the foundation for unlocking the high-strength, high-conductivity potential of copper alloys.
Summary Table:
| Process Stage | Action Taken | Microstructural Change | Key Objective |
|---|---|---|---|
| High-Temp Heating | Heat above solvus line | Cr and Cu5Zr dissolve into Cu matrix | Create supersaturated solid solution |
| Water Quenching | Rapid cooling | Atomic structure is "frozen" | Prevent premature precipitation |
| Resulting State | Ambient cooling | Metastable single-phase structure | Maximize potential for age hardening |
Unlock the Potential of Your Copper Alloys with KINTEK
Achieving the perfect metastable state for Cu-Cr-Zr-La alloys requires uncompromising thermal precision. At KINTEK, we understand that even a slight temperature deviation or quench delay can compromise your material's conductivity and hardness.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Our lab high-temp furnaces are fully customizable to meet the rigorous solvus line requirements of your unique research or production needs.
Ready to elevate your heat treatment process? Contact our technical experts today to find the ideal furnace solution for your laboratory.
Visual Guide
References
- Hairui Zhi, Haitao Zhao. Low cycle fatigue behavior of Cu-Cr-Zr-La alloys. DOI: 10.1088/1742-6596/2951/1/012133
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is a laboratory vacuum evaporation system essential for the preparation of electrodes in high-performance solar cells?
- Why is a vacuum drying oven necessary for sample pretreatment in iodine gas capture experiments?
- What is the purpose of treating ADSC powders with hydrogen? Purify Your Material for Superior Conductivity
- What is the function of a laboratory cryofurnace during Co3O2BO3 experiments? Precise Phase Transition Control
- What role does the annealing process play in the post-treatment of stir-cast aluminum matrix composites? | KINTEK
- How does a precision pressure-controlled oxidation device increase carbon chain yield? Optimize Your Annealing Process
- What are the advantages of using a vacuum drying oven for purifying zinc oxide nanoparticles? Superior Material Quality
- What role does Sodium Chloride (NaCl) play as a thermal buffer? Optimizing Si/Mg2SiO4 Composite Synthesis



















