A precision pressure-controlled oxidation device acts as a specific feedstock regenerator within the multi-step annealing process. By subjecting the material to a precise environment of 500°C and 600 mbar of air pressure, the device selectively etches residual nanotube structures and opens internal caps. This etching process liberates free carbon atoms, which are then repurposed as the raw building blocks necessary for continued growth in the next stage, directly resulting in a significant increase in the bulk yield of carbon chains.
The device functions not just as a cleaning tool, but as a carbon recycling system. By converting structural byproducts into usable free atoms, it ensures the synthesis process has the fuel required to maximize chain formation.
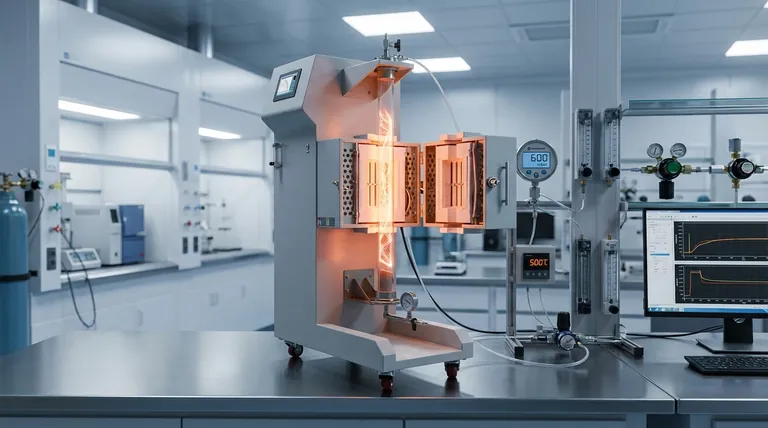
The Mechanics of Yield Enhancement
Targeted Structural Breakdown
The device operates at a specific intermediate condition, maintained strictly at 500°C and 600 mbar.
Under these conditions, the oxidation preferentially attacks residual single-walled carbon nanotube sections. This removes unwanted structural debris that might otherwise hinder the synthesis process.
Opening the Internal Architecture
Beyond removing debris, the process plays a constructive role by targeting the caps of newly formed internal tube walls.
The oxidation effectively "uncaps" these structures. This opening is a prerequisite for further chemical interaction and growth within the nanotube assembly.
The Carbon Regeneration Cycle
Creating "Free" Carbon
The physical etching of the nanotubes and caps is not a destructive end-state; it is a generative process.
As the oxidation breaks down these carbon structures, it releases free carbon atoms. These atoms are no longer bound to the rigid tube lattice.
Fueling Subsequent Growth
These liberated atoms serve as immediate raw material (feedstock) for the next annealing stage.
Instead of introducing external carbon sources exclusively, the system recycles this etched material. This abundance of available carbon fuels the subsequent growth phase, driving the significant increase in bulk yield.
Critical Process Constraints
The Importance of Precision
The effectiveness of this technique relies entirely on the stability of the pressure and temperature parameters.
The specific setting of 600 mbar is calibrated to balance etching with preservation. If the pressure is too high, the oxidation may become too aggressive, destroying the carbon chains rather than just the residual sections.
Temperature Sensitivity
Similarly, the 500°C thermal environment must be maintained to ensure the release of carbon atoms without compromising the structural integrity of the primary chains.
A deviation in temperature could result in a failure to open the tube caps, effectively starving the next stage of its necessary feedstock.
Making the Right Choice for Your Goal
To maximize the benefits of this intermediate treatment, align your process controls with your specific objectives:
- If your primary focus is Maximizing Yield: Strictly maintain the 600 mbar pressure to ensure the maximum amount of "waste" carbon is converted into usable feedstock for the next stage.
- If your primary focus is Structural Integrity: Closely monitor the 500°C limit to ensure the etching targets only residual sections and caps, preventing damage to the primary carbon chains.
Success in this process comes from viewing oxidation not as a removal step, but as a vital transformation step that fuels production.
Summary Table:
| Process Parameter | Target Condition | Role in Yield Enhancement |
|---|---|---|
| Temperature | 500°C | Enables selective etching without damaging primary carbon chains. |
| Air Pressure | 600 mbar | Balances oxidation to uncap structures and release free carbon atoms. |
| Mechanism | Feedstock Regeneration | Converts structural byproducts/debris into usable raw building blocks. |
| Primary Result | Bulk Yield Increase | Provides high-density carbon fuel for subsequent growth stages. |
Maximize Your Lab’s Synthesis Efficiency with KINTEK
Precise control over thermal and pressure environments is the difference between structural failure and high-yield success. At KINTEK, we understand the nuances of carbon chain synthesis and advanced material growth. Backed by expert R&D and manufacturing, we offer industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain the exacting tolerances your research demands.
Whether you need a specialized oxidation environment or a fully customizable high-temperature furnace, KINTEK provides the reliability and precision to transform your byproducts into performance.
Ready to scale your results? Contact our technical experts today to find the perfect solution for your unique annealing needs.
Visual Guide
References
- Clara Freytag, Thomas Pichler. Systematic Optimization of the Synthesis of Confined Carbyne. DOI: 10.1002/smtd.202500075
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What role does quartz sand filler play in a crystal growth furnace? Enhance Thermal Symmetry and Yield
- Why is temperature control precision critical for a sample heating furnace? Master Ti-V-Cr Alloy Oxidation Kinetics
- What type of furnace is used for heat treatment? Choose the Right Solution for Your Materials
- Why is a stainless steel high-pressure autoclave essential for starch hydrogenation? Unlock Peak Reaction Efficiency
- Why is 700°C Pre-treatment Necessary for D2O Hydration on Ba0.95La0.05(Fe1-xYx)O3-δ? Ensuring Accurate Results
- What physical environment does a laboratory oven provide for perovskite annealing? Master Thermal Precision & Strain Control
- What role does the vitreous carbon foam framework play in PTTM? Unlock Biomimetic Dental Implant Precision
- Why is a vacuum heating pretreatment system essential for zeolite characterization? Ensure Precise Pore Structure Data



















