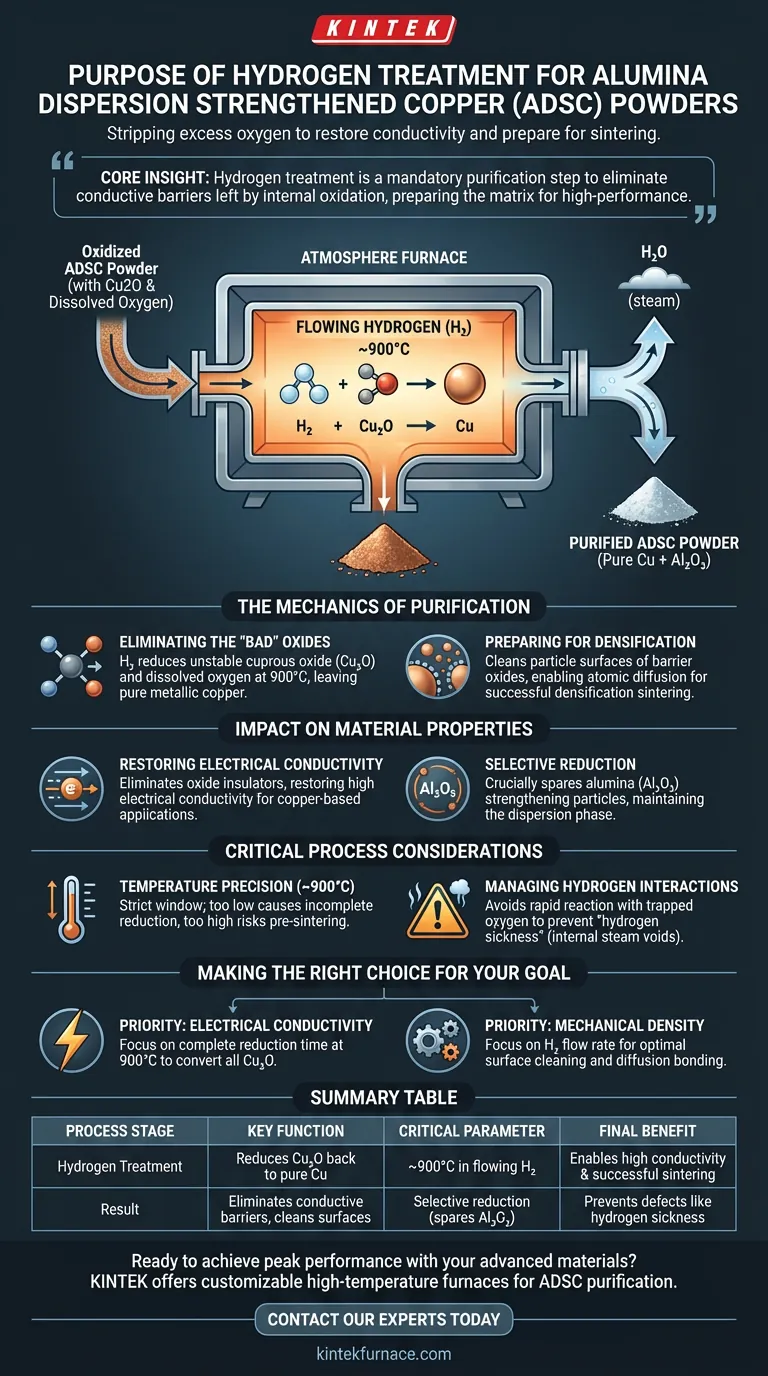
The primary purpose of treating ADSC powders with hydrogen is to chemically reduce the material, stripping away excess oxygen introduced during the internal oxidation phase. By maintaining a temperature of approximately 900°C in flowing hydrogen, this process converts unwanted copper oxides back into pure metallic copper without disturbing the strengthening alumina particles.
Core Insight: While internal oxidation is vital for creating the strengthening alumina phase, it leaves behind "collateral damage" in the form of dissolved oxygen and copper oxides. The hydrogen treatment is a mandatory purification step designed to eliminate these conductive barriers, preparing the matrix for high-performance applications.

The Mechanics of Purification
Eliminating the "Bad" Oxides
Following internal oxidation, the copper matrix is saturated with excess oxidants and dissolved oxygen, often manifesting as cuprous oxide (Cu2O).
The atmosphere furnace uses flowing hydrogen gas to act as a reducing agent. At temperatures around 900°C, the hydrogen reacts with these unstable copper oxides, effectively removing the oxygen and leaving behind pure metallic copper.
Preparing for Densification
The presence of surface oxides on powder particles acts as a barrier to atomic diffusion.
By purifying the matrix and cleaning the particle surfaces, this heat treatment ensures the material is chemically active for the next stage of manufacturing. This removal of impurities is a prerequisite for successful densification sintering, allowing the particles to bond effectively into a solid mass.
Impact on Material Properties
Restoring Electrical Conductivity
Dissolved oxygen and copper oxide inclusions significantly degrade the electrical performance of the final product.
Because copper oxides act as electrical insulators, they disrupt the flow of electrons through the matrix. The hydrogen reduction step eliminates these scattering sites, ensuring the final ADSC material achieves the high electrical conductivity expected of copper-based alloys.
Selective Reduction
It is critical to note that this process is selective.
While hydrogen effectively reduces copper oxides, it does not reduce the aluminum oxide (alumina) particles generated during the previous internal oxidation step. This ensures the material retains its dispersion-strengthening phase (the alumina) while cleaning the matrix (the copper).
Critical Process Considerations
Temperature Precision
The process relies on maintaining a strict temperature window, typically around 900°C.
Deviating significantly from this temperature can compromise the process. Temperatures that are too low may result in incomplete reduction, leaving residual oxides that hamper performance, while excessive heat could lead to unwanted pre-sintering or grain coarsening before the densification stage.
Managing Hydrogen Interactions
While hydrogen is the cleaning agent, it must be managed carefully to avoid material defects.
If hydrogen reacts with oxygen trapped deep within the copper lattice too rapidly, it can form high-pressure water vapor (steam). This phenomenon, often called hydrogen sickness, can create internal voids or fissures, undermining the structural integrity of the copper matrix.
Making the Right Choice for Your Goal
This reduction step is the bridge between creating the strengthening phase and consolidating the final material. Here is how to prioritize your process parameters:
- If your primary focus is Electrical Conductivity: Prioritize complete reduction time at 900°C to ensure every trace of Cu2O is converted to metallic copper, as even minor oxide residues will increase resistivity.
- If your primary focus is Mechanical Density: Focus on the flow rate of hydrogen to ensure optimal surface cleaning of the particles, which maximizes diffusion bonding during the subsequent sintering phase.
Ultimately, the hydrogen treatment transforms a chemically contaminated intermediate powder into a pure, conductive, and sinter-ready engineering material.
Summary Table:
| Process Stage | Key Function | Critical Parameter |
|---|---|---|
| Hydrogen Treatment | Reduces copper oxides (Cu₂O) back to pure copper | ~900°C in flowing H₂ |
| Result | Eliminates conductive barriers, cleans particle surfaces | Selective reduction (spares Al₂O₃) |
| Final Benefit | Enables high electrical conductivity and successful sintering | Prevents defects like hydrogen sickness |
Ready to achieve peak performance with your advanced materials?
The hydrogen treatment process is critical for transforming ADSC powders into high-performance components. Ensuring precise temperature control and atmosphere management is key to success.
Backed by expert R&D and manufacturing, KINTEK offers Tube, Vacuum, and other lab high-temperature furnaces, all customizable for unique thermal processing needs like ADSC purification.
Let's optimize your process. Contact our experts today to discuss your application requirements!
Visual Guide
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What is the purpose of vacuum sputtering systems for haptic device electrodes? Achieve High-Precision Metal Deposition
- Why is high temperature control precision essential for SiC/SiC composites? Master Microstructural Engineering
- How does Vacuum Degassing (VD) influence spinel inclusions in heavy rail steel? Controlling Refractory Erosion
- How does the placement of copper foil affect single-crystal Cu(111) preparation? Achieve Perfect Grain Growth
- What is a horizontal furnace? A space-saving heating solution for attics and crawl spaces
- What is the function of wet ball milling in the synthesis of SPAN? Optimize Your Sulfur Content Through Deep Mixing
- What heat treatment conditions are required for SDSS2507 solution treatment? Achieve Precise 1100°C Thermal Profiles
- What is the purpose of the long-term stabilization sintering step at 250°C? Secure Your CuO Nano-Network Integrity



















