Sodium Chloride (NaCl) serves as a critical thermal regulator in the synthesis of Silicon/Magnesium Silicate composites. By acting as a chemically stable buffer, it absorbs and redistributes the intense heat generated during the pre-magnesiation phase, preventing localized overheating and ensuring the reaction proceeds uniformly.
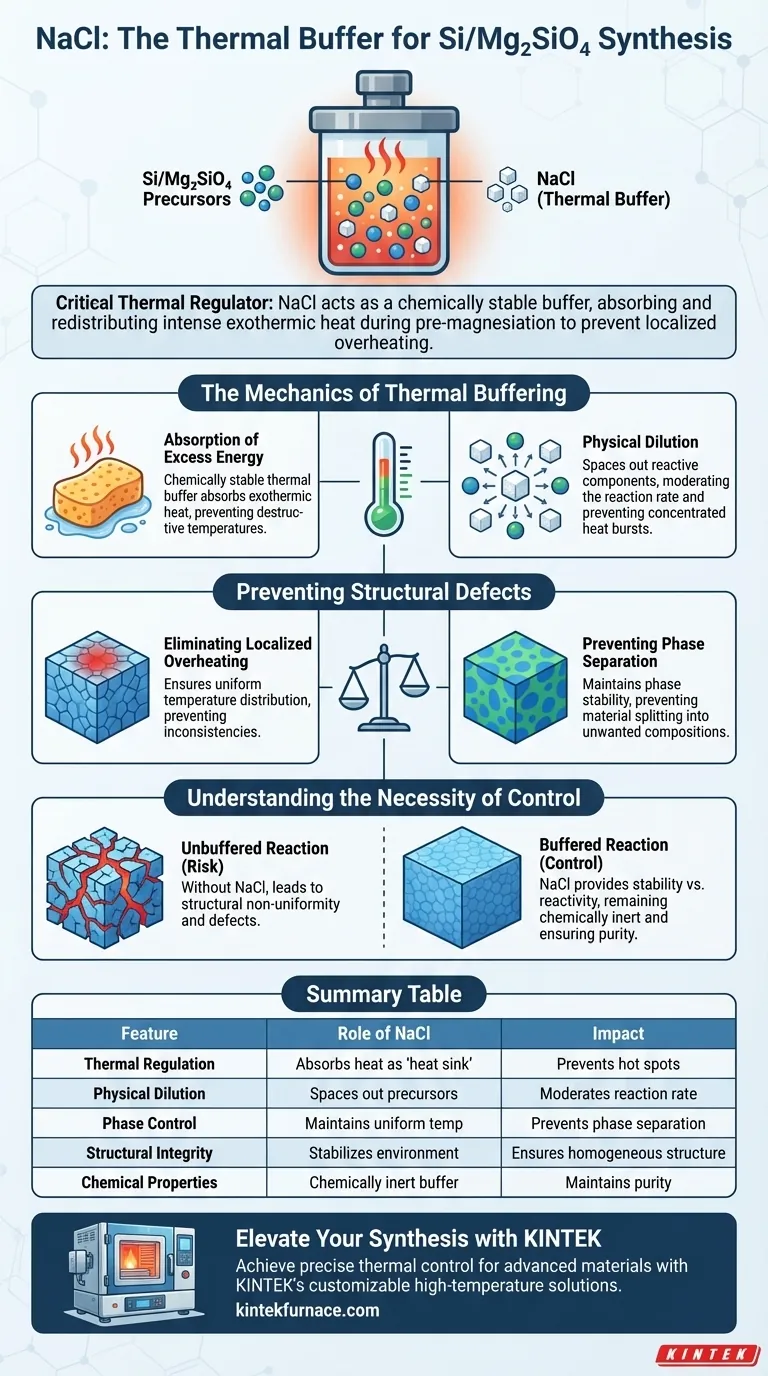
The Core Mechanism The pre-magnesiation reaction is highly exothermic, creating risks of structural failure. NaCl acts as a "heat sink" and physical diluent, absorbing excess energy to preserve the structural integrity and phase uniformity of the final composite.

The Mechanics of Thermal Buffering
Absorption of Excess Energy
During the synthesis process, specifically the pre-magnesiation reaction, significant heat is generated. NaCl is mixed into the precursor powder to serve as a chemically stable thermal buffer.
It functions by absorbing this excess thermal energy. By soaking up the heat, it prevents the reaction environment from reaching uncontrolled, destructive temperatures.
Physical Dilution
Beyond simple heat absorption, NaCl provides physical dilution within the mixture. By spacing out the reactive components, it moderates the reaction rate.
This separation ensures that heat is not generated in concentrated bursts that the material cannot dissipate.
Preventing Structural Defects
Eliminating Localized Overheating
Without a buffer, the exothermic nature of the reaction can lead to localized hot spots. These temperature spikes create inconsistencies within the material.
NaCl ensures a uniform temperature distribution throughout the powder mixture. This thermal balance is essential for consistent material quality.
Preventing Phase Separation
Temperature uniformity is directly linked to phase stability. Localized overheating can cause phase separation, where the material splits into unwanted chemical compositions.
By maintaining a stable thermal environment, NaCl ensures the Silicon/Magnesium Silicate composite retains a homogeneous structure.
Understanding the Necessity of Control
The Risk of Unbuffered Reactions
It is a common pitfall to underestimate the intensity of the pre-magnesiation reaction. Omitting a thermal buffer like NaCl often results in structural non-uniformity.
If the heat is not dispersed, the final composite will likely suffer from defects that compromise its performance.
Stability vs. Reactivity
The inclusion of NaCl strikes a balance between reactivity and control. While the goal is to synthesize the composite, the chemical stability of NaCl ensures it does not interfere with the desired reaction.
It participates physically (as a spacer and heat sink) but remains chemically inert, ensuring the purity of the final Si/Mg2SiO4 product is not compromised by side reactions.
Achieving Optimal Synthesis Results
To maximize the quality of your Silicon/Magnesium Silicate composites, the application of the thermal buffer must be strategic.
- If your primary focus is Structural Homogeneity: Ensure the NaCl is thoroughly mixed into the precursor powder to prevent any localized hot spots during heating.
- If your primary focus is Phase Purity: Rely on the physical dilution effect of NaCl to keep reaction temperatures below the threshold where phase separation occurs.
By effectively utilizing NaCl as a thermal buffer, you convert a volatile exothermic reaction into a controlled, uniform synthesis process.
Summary Table:
| Feature | Role of NaCl in Synthesis | Impact on Final Composite |
|---|---|---|
| Thermal Regulation | Absorbs exothermic heat as a 'heat sink' | Prevents localized hot spots |
| Physical Dilution | Spaces out reactive precursors | Moderates reaction rate and intensity |
| Phase Control | Maintains uniform temperature environment | Prevents phase separation and impurities |
| Structural Integrity | Stabilizes the reaction environment | Ensures homogeneous material structure |
| Chemical Properties | Chemically inert buffer | Maintains purity without side reactions |
Elevate Your Advanced Material Synthesis with KINTEK
Achieving precise phase purity and structural homogeneity in Silicon/Magnesium Silicate composites requires more than just the right buffer—it requires exact thermal control.
Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance laboratory solutions including Muffle, Tube, Rotary, Vacuum, and CVD systems. Our high-temperature furnaces are fully customizable to meet the unique exothermic challenges of your research, ensuring your synthesis process is consistent, safe, and scalable.
Ready to optimize your lab's thermal processing? Contact our experts today to find your custom furnace solution.
Visual Guide
References
- Hyunsik Yoon, Hansu Kim. Magnesiated Si‐Rich SiO<sub><i>x</i></sub> Materials for High‐Performance Lithium‐Ion Batteries. DOI: 10.1002/batt.202500473
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What are the primary functional zones of a conveyor belt furnace? Optimize Your Copper Brazing Process
- What industries commonly use batch furnaces? Essential for Aerospace, Medical, and Electronics
- How is the influence of permeation temperature on steel hardness quantified? Precision Modeling for Plasma Nitriding
- How does a vacuum oven contribute to the performance of composite electrode slurries? Enhance Battery Life & Stability
- What are the energy consumption considerations when choosing between separate or combined debinding and sintering furnaces? Optimize Your Process Efficiency
- Why is titanium used as a gettering agent in TiCo1-xCrxSb preparation? Achieve Purity in Your Alloy Synthesis
- Why is a precision oven used to dry washed cherry pits? Unlock Superior Activated Carbon Production
- What are the advantages of using a vacuum oven for drying VO2@AlF3? Protect Your Sensitive Core-Shell Nanostructures



















