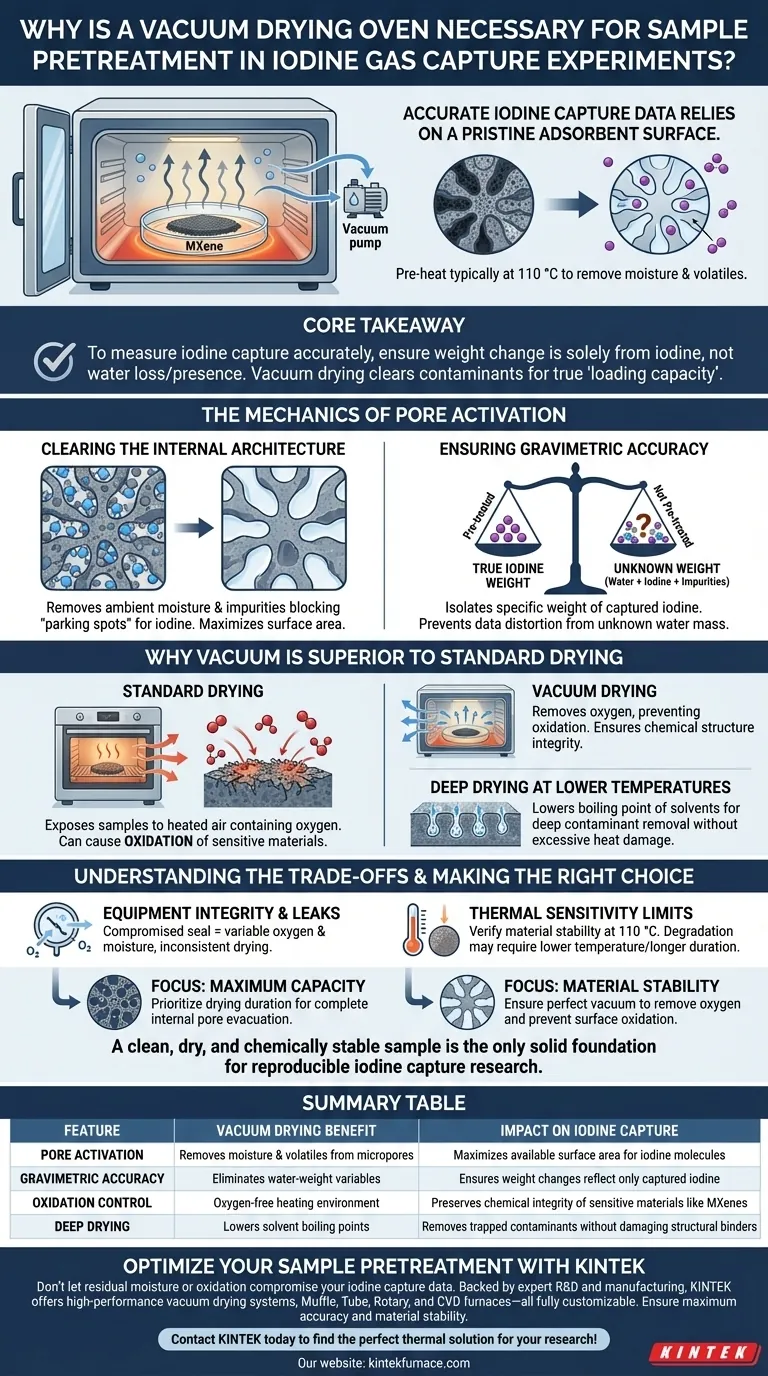
Accurate iodine capture data relies on a pristine adsorbent surface. A vacuum drying oven is necessary to pre-heat samples (typically at 110 °C) to rigorously remove residual moisture and volatile impurities that are physically adsorbed within the internal pores of materials like MXene. This step effectively "resets" the material, ensuring that pore sites are open for capture and eliminating water weight that would otherwise distort the calculation of static iodine loading capacity.
Core Takeaway To measure iodine capture accurately, you must ensure the material's weight change is caused solely by iodine, not by the loss or presence of water. Vacuum drying clears the internal pore structure of contaminants, guaranteeing that the "loading capacity" reflects the material's true performance.

The Mechanics of Pore Activation
Clearing the Internal Architecture
Adsorbent materials, such as MXenes, rely on complex internal pore structures to trap gas. However, these micropores often act as traps for ambient moisture and volatile impurities.
If these impurities remain, they physically block the "parking spots" where iodine molecules are meant to sit. Vacuum drying effectively evacuates these pores, maximizing the surface area available for the experiment.
Ensuring Gravimetric Accuracy
The "static iodine loading capacity" is typically calculated based on weight change.
If a sample is not pre-treated, it contains an unknown mass of water. During the experiment, this water might evaporate while iodine is being adsorbed, or it might remain and be counted as part of the sample's base weight. Either scenario makes it impossible to isolate the specific weight of the captured iodine, rendering the data invalid.
Why Vacuum is Superior to Standard Drying
Preventing Material Oxidation
While the primary goal is moisture removal, the method matters. Standard drying ovens expose samples to heated air, which contains oxygen.
A vacuum environment removes oxygen from the chamber. This is critical for preventing the oxidation of sensitive materials (like MXenes or specific electrode components) that might degrade when heated in air. It ensures the chemical structure of the adsorbent remains intact before the experiment begins.
Deep Drying at Lower Temperatures
Vacuum drying lowers the pressure inside the chamber, which consequently lowers the boiling point of solvents and moisture.
This allows for "deep drying"—removing stubborn solvents trapped deep within micropores—without requiring excessive temperatures that could damage the material's binder or structural framework. It prevents the phenomenon of "surface hardening," where rapid surface drying traps moisture inside the core of the sample.
Understanding the Trade-offs
Equipment Integrity and Leaks
The effectiveness of this process is entirely dependent on the quality of the vacuum seal. A compromised seal introduces a variable amount of oxygen and moisture back into the chamber, which can lead to inconsistent drying and unexpected oxidation, defeating the purpose of the pretreatment.
Thermal Sensitivity Limits
While vacuum drying lowers the boiling point of water, the standard protocol often calls for 110 °C. You must verify that your specific adsorbent material is thermally stable at this temperature. If the material degrades at 110 °C, the vacuum alone cannot save the sample, and a lower temperature with a longer duration may be required.
Making the Right Choice for Your Experiment
To ensure your iodine capture data is reproducible and valid, consider your specific experimental goals:
- If your primary focus is Maximum Capacity: Prioritize the duration of the drying phase to ensure deep internal pores are completely evacuated of moisture.
- If your primary focus is Material Stability: Ensure the vacuum pump is functioning perfectly to remove oxygen, preventing surface oxidation that could alter the material's chemical reactivity.
A clean, dry, and chemically stable sample is the only solid foundation for reproducible iodine capture research.
Summary Table:
| Feature | Vacuum Drying Benefit | Impact on Iodine Capture |
|---|---|---|
| Pore Activation | Removes moisture & volatiles from micropores | Maximizes available surface area for iodine molecules |
| Gravimetric Accuracy | Eliminates water-weight variables | Ensures weight changes reflect only captured iodine |
| Oxidation Control | Oxygen-free heating environment | Preserves chemical integrity of sensitive materials like MXenes |
| Deep Drying | Lowers solvent boiling points | Removes trapped contaminants without damaging structural binders |
Optimize Your Sample Pretreatment with KINTEK
Don’t let residual moisture or oxidation compromise your iodine capture data. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum drying systems, Muffle, Tube, Rotary, and CVD furnaces—all fully customizable to meet your laboratory’s unique high-temperature needs. Ensure maximum accuracy and material stability in every experiment.
Contact KINTEK today to find the perfect thermal solution for your research!
Visual Guide
References
- Karamullah Eisawi, Michael Naguib. Nanohybrid of Silver‐MXene: A Promising Sorbent for Iodine Gas Capture from Nuclear Waste. DOI: 10.1002/admi.202500011
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Induction Melting Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- Why is MFI-type zeolite (S-1) selected for H-TiO2 synthesis? Master High-Efficiency Nanoparticle Templating
- What is induction heating and what materials can it be used on? A Guide to Fast, Precise Heating
- How does plasma nitriding equipment improve the performance of titanium alloys in seawater? Boost Marine Durability
- Why is rapid water quenching necessary for Ce2(Fe, Co)17 alloys? Unlock Peak Magnetocaloric Performance
- What is the function of rapid quenching after high-temperature heat treatment? Master AlSi10Mg Microstructural Control
- What is the function of a rotary high-pressure autoclave in the synthesis of SSZ-13 zeolites? | Enhance Crystallinity
- Why is long-term NaOH immersion required for porous carbon? Optimize Your Template Removal & Surface Area
- Why is continuous removal of carbon essential for methane pyrolysis reactors? Protect Your Reactor's Integrity



















