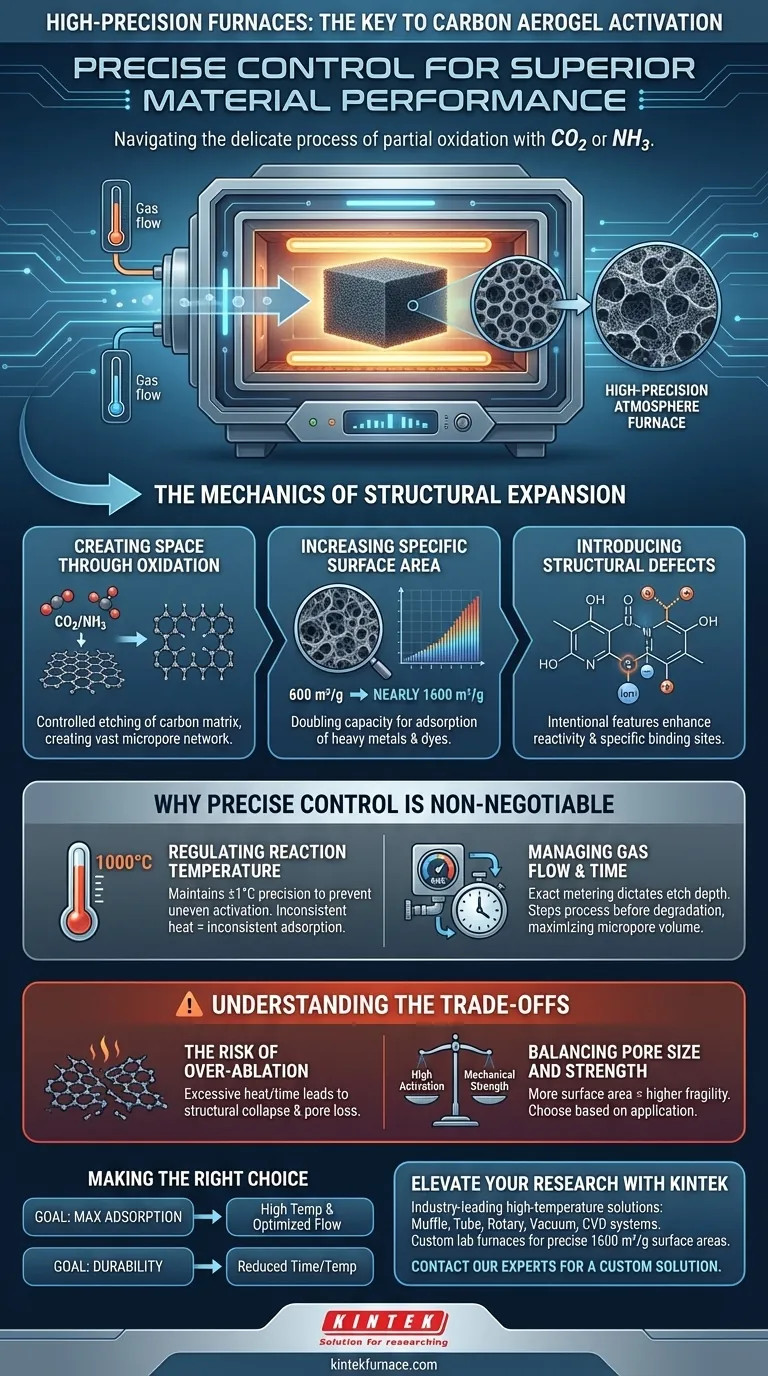
Precise environmental control is the deciding factor in successful aerogel activation. A high-precision temperature-controlled atmosphere furnace is required to facilitate "partial oxidation," a delicate process where activation agents like carbon dioxide or ammonia etch the carbon matrix. By strictly regulating reaction temperature, gas flow, and time, the furnace ensures the material creates new micropores without destroying its structural integrity.
The core purpose of this furnace is to achieve controlled ablation. It allows the operator to selectively etch the carbon skeleton, transforming a standard aerogel into a high-performance material with vastly increased surface area and adsorption capacity.

The Mechanics of Structural Expansion
Creating Space Through Oxidation
The activation process is fundamentally a subtractive method. The furnace uses high temperatures to induce a reaction between the carbon aerogel and the activating gas ($CO_2$ or $NH_3$).
This reaction causes partial oxidation, effectively "eating away" specific parts of the carbon structure. This etching process creates a vast network of micropores within the material.
Increasing Specific Surface Area
The primary goal of this treatment is to maximize the physical space available for adsorption. Without the precise environment provided by the furnace, the material would not develop the necessary internal surface area.
Data indicates that proper activation can more than double the specific surface area, potentially jumping from roughly 600 m²/g to nearly 1600 m²/g. This expansion is critical for applications involving the capture of heavy metal ions or dye molecules.
Introducing Structural Defects
Beyond simple pore creation, the furnace environment fosters physical and chemical activation that introduces structural defects.
These defects are not failures; they are intentional features that enhance the material's reactivity. They provide specific binding sites that significantly improve the aerogel's ability to adsorb contaminants like mercury from aqueous solutions.
Why Precise Control is Non-Negotiable
Regulating Reaction Temperature
Activation often requires extreme heat, such as 1000 °C for carbon dioxide activation. The furnace must maintain this temperature with high precision to ensure the reaction occurs uniformly throughout the batch.
Fluctuations in temperature can lead to uneven activation, resulting in a product with inconsistent adsorption performance.
Managing Gas Flow and Time
The duration of the treatment and the flow rate of the activation gas are just as critical as the temperature. The furnace allows for the exact metering of these variables.
This control dictates the "depth" of the etch. It ensures the process stops exactly when the micropore volume is maximized, rather than allowing the reaction to continue until the material degrades.
Understanding the Trade-offs
The Risk of Over-Ablation
While the goal is to etch the material, there is a fine line between activation and destruction. If the furnace temperature is too high or the exposure time too long, the oxidation becomes aggressive.
This leads to structural collapse, where the carbon skeleton is consumed entirely. The result is a loss of mechanical strength and a paradoxically lower surface area because the pore walls have burned away.
balancing Pore Size and Strength
High activation creates more surface area but yields a more fragile material. A highly activated aerogel is excellent for static adsorption tasks but may lack the mechanical robustness required for high-stress filtration environments.
Making the Right Choice for Your Goal
To optimize your activation process, consider your end-use application:
- If your primary focus is maximum adsorption capacity: Prioritize higher temperatures (around 1000 °C) and optimized gas flow to maximize micropore volume and specific surface area.
- If your primary focus is structural durability: Reduce the reaction time or temperature slightly to preserve a thicker carbon skeleton, accepting a moderate reduction in total surface area.
Mastering the variables of temperature and time turns a simple carbon material into a highly efficient molecular sponge.
Summary Table:
| Activation Parameter | Role in Process | Impact on Material Performance |
|---|---|---|
| Temperature (up to 1000°C) | Facilitates partial oxidation/etching | Regulates reaction rate and uniform pore creation |
| Gas Flow ($CO_2$ / $NH_3$) | Acts as the activating agent | Controls depth of etch and introduction of defects |
| Reaction Time | Manages duration of ablation | Balances maximum surface area vs. structural integrity |
| Atmosphere Control | Prevents unwanted combustion | Ensures specific binding sites for heavy metal adsorption |
Elevate Your Material Research with KINTEK
Don't let inconsistent thermal environments compromise your aerogel's performance. KINTEK provides industry-leading high-temperature solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all precision-engineered for delicate processes like carbon activation. Our customizable lab furnaces ensure the exact temperature and atmosphere control required to achieve surface areas of 1600 m²/g without structural collapse.
Ready to optimize your activation process? Contact our expert R&D team today to find your custom solution.
Visual Guide
References
- Yong Zhong, Xuguang Liu. Carbon Aerogel for Aqueous Phase Adsorption/Absorption: Application Performances, Intrinsic Characteristics, and Regulatory Constructions. DOI: 10.1002/sstr.202400650
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is a vacuum desiccator used for the preservation of extracted fruit peel extracts? Protect Bioactive Compounds
- What is the specific purpose of using a laboratory oven for the treatment of copper oxide precipitates? Expert Insights
- What is a batch furnace and how does it operate? Master Precision Heat Treatment for Diverse Applications
- What is the function of an inert gas supply system in black liquor pyrolysis? Achieve Precise Atmospheric Control
- Why is a constant temperature drying oven set to 60°C for 24 hours? Optimizing Sr4Al6O12SO4 Powder Quality
- What are the main types of sintering furnaces? Find the Perfect Match for Your Materials
- What is preventive maintenance on a furnace? A Proactive Strategy for Peak Performance
- What is the purpose of post-treating Nitrogen-doped Carbide-Derived Carbon (N-CDC)? Optimize Purity & Performance



















