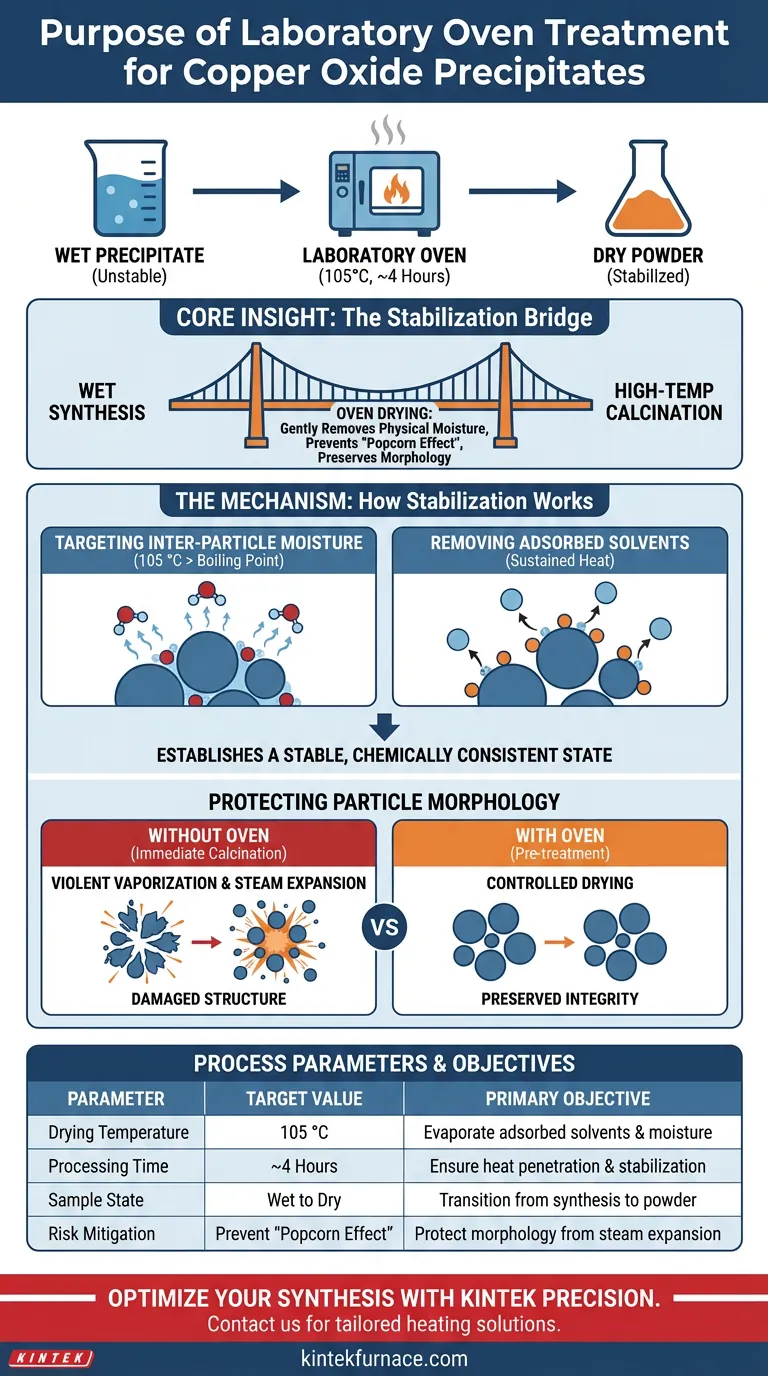
The primary specific purpose of using a laboratory oven for copper oxide precipitates is to remove physically adsorbed solvents and inter-particle moisture through a controlled drying process. By subjecting the washed wet precipitates to a constant temperature of 105 °C for approximately 4 hours, the oven ensures the material is thoroughly dried and stabilized before it undergoes further thermal processing.
Core Insight: The laboratory oven acts as a critical stabilization bridge between wet synthesis and high-temperature calcination. Its main function is to gently eliminate physical moisture to prevent the "popcorn effect"—violent vaporization that destroys particle morphology—during subsequent high-heat treatment.
The Mechanism of Sample Stabilization
Targeting Inter-particle Moisture
The laboratory oven operates at a specific set point of 105 °C. This temperature is slightly above the boiling point of water, ensuring the efficient evaporation of moisture trapped between particles.
Removing Adsorbed Solvents
Beyond simple water, the process targets "physically adsorbed solvents." These are liquids adhering to the surface of the precipitate that require sustained heat to dislodge fully.
Establishing a Stable State
The 4-hour duration is not arbitrary; it allows sufficient time for heat to penetrate the sample core. This results in a chemically consistent, dry powder that is stable enough for handling and analysis.
Protecting Particle Morphology
Preventing Violent Vaporization
If a wet sample is immediately exposed to the extreme heat of calcination, the trapped water effectively flashes into steam. This rapid expansion creates internal pressure.
Preserving Structural Integrity
The primary reference highlights that this internal pressure can cause the physical destruction of the particle's shape. By removing moisture gently in the oven first, you avoid these micro-explosions and preserve the intended morphology of the copper oxide.
Preparing for Calcination
The oven drying step effectively "pre-treats" the sample. It ensures that the subsequent high-temperature calcination focuses solely on phase transformation and crystallization, rather than water removal.
Understanding the Trade-offs
Time vs. Throughput
The 4-hour requirement at 105 °C creates a bottleneck in processing speed. Attempting to shorten this time can lead to residual moisture, which endangers the sample during the next stage.
Temperature Precision
Setting the oven significantly higher than 105 °C to speed up drying is risky. Excessive heat at this stage could trigger premature chemical changes or oxidation before the sample is physically ready.
Ensuring Process Consistency
To maximize the quality of your copper oxide treatment, apply the drying process strategically based on your downstream requirements.
- If your primary focus is preserving particle shape: Adhere strictly to the low-temperature (105 °C) drying phase to eliminate the risk of structural collapse caused by steam expansion.
- If your primary focus is process reproducibility: Maintain the standard 4-hour duration to ensure that every batch enters the calcination furnace with the exact same low-moisture profile.
Proper oven drying is the fundamental safeguard that ensures a wet precipitate successfully transitions into a high-quality ceramic precursor.
Summary Table:
| Process Parameter | Target Value | Primary Objective |
|---|---|---|
| Drying Temperature | 105 °C | Evaporate adsorbed solvents and moisture |
| Processing Time | ~4 Hours | Ensure heat penetration and stabilization |
| Sample State | Wet Precipitate | Transition from wet synthesis to dry powder |
| Risk Mitigation | Prevent 'Popcorn Effect' | Protect particle morphology from steam expansion |
Optimize Your Synthesis with KINTEK Precision
Don’t let moisture compromise your material integrity. Backed by expert R&D and manufacturing, KINTEK offers high-performance laboratory ovens and specialized high-temperature systems—including Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable for your unique lab requirements.
Whether you are treating copper oxide precipitates or engineering advanced ceramic precursors, our equipment ensures the precise temperature control and uniformity you need for reproducible results. Contact us today to discuss your project needs and see how our tailored heating solutions can elevate your research and production.
Visual Guide
References
- Charlena Charlena, Dila Ardiansyah. Synthesis and Characterization of Copper(II) Oxide (CuO-NP) Nanoparticles using Chemical Precipitation Method. DOI: 10.30872/jkm.v21i2.1260
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the role of a dedicated bias power supply in low-pressure plasma nitriding? Master Ion Acceleration Control
- How do laboratory high-temperature furnaces facilitate the control of nano-scale TiC and VC precipitates? | KINTEK
- What critical environment does a high-temp furnace provide for H13 steel? Mastering Microstructural Homogenization
- How does the combination of a nitrogen atmosphere and magnetic stirring benefit the dissolution stage? | KINTEK
- Why are raw materials compacted into briquettes for vacuum carbothermal reduction? Optimize Your Magnesium Production
- How does the SCRS model simplify furnace combustion simulation? Efficiency Meets Accuracy in Thermal Modeling
- What are the core technical advantages of single-step microwave furnace sintering for SSBSN ceramics?
- Why is the high-precision control of argon (Ar) and nitrogen (N2) flow ratios critical in CrSiN-Y coating fabrication?



















