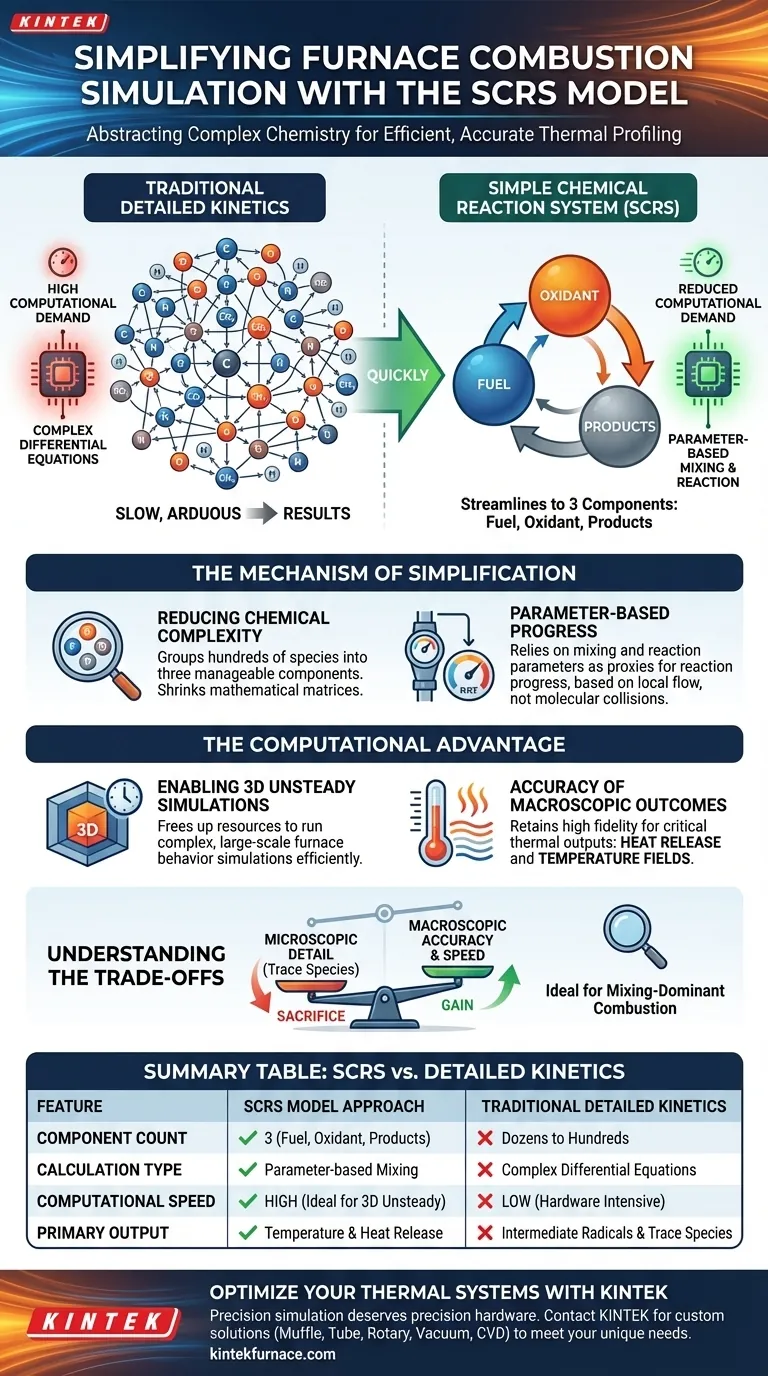
The Simple Chemical Reaction System (SCRS) model streamlines numerical simulations by abstracting complex combustion chemistry into a fundamental interaction between just three components: fuel, oxidant, and products. Instead of calculating the intricate behaviors of numerous intermediate chemical species, SCRS utilizes mixing and reaction parameters to describe the process, significantly reducing computational demand while maintaining accuracy in temperature and heat release predictions.
The SCRS model solves the computational bottleneck of combustion simulation by prioritizing macroscopic mixing over microscopic chemical details. It allows for efficient 3D unsteady simulations without sacrificing the accuracy of the resulting thermal fields.

The Mechanism of Simplification
Reducing Chemical Complexity
In detailed combustion simulations, a solver must typically track dozens or even hundreds of intermediate chemical species and their reactions.
The SCRS model bypasses this by grouping the entire system into three manageable components: fuel, oxidant, and products. This reduction drastically shrinks the size of the mathematical matrices the solver must process at every time step.
Parameter-Based Progress
Rather than solving differential equations for every chemical kinetic step, SCRS relies on mixing and chemical reaction parameters.
These parameters act as proxies for the reaction progress. They allow the simulation to determine how much fuel has been consumed and how much heat has been released based on local flow and mixing conditions, rather than molecular-level collision rates.
The Computational Advantage
Enabling 3D Unsteady Simulations
Simulating a furnace in three dimensions over time (unsteady simulation) is computationally expensive.
By removing the "stiff" equations associated with detailed chemical kinetics, SCRS frees up computational resources. This makes it feasible to run complex, large-scale simulations of furnace behavior that would otherwise be too slow or hardware-intensive to solve.
Accuracy of Macroscopic Outcomes
Despite the simplification, the model retains high fidelity where it counts for furnace engineering: heat release and temperature fields.
The primary reference indicates that for predicting the thermal environment—which is the primary function of a furnace—the interaction between the three core components provides sufficient data to generate accurate results.
Understanding the Trade-offs
The Sacrifice of Microscopic Detail
The efficiency of the SCRS model comes from ignoring detailed chemical kinetics.
While excellent for thermal profiling, this approach does not explicitly model the formation of intermediate radicals or complex trace species. You are trading chemical granularity for computational speed.
Scope of Applicability
This model is ideal for scenarios where the physical mixing of fuel and air is the dominant factor in combustion.
However, if your simulation requires precise tracking of slow-forming pollutants or ignition delays dependent on specific chemical chains, the simplified three-component approach may require careful validation.
Making the Right Choice for Your Simulation
To determine if the Simple Chemical Reaction System is the right tool for your furnace simulation, consider your specific engineering goals.
- If your primary focus is Thermal Profiling: The SCRS model is highly recommended as it accurately predicts temperature fields and heat release with minimal computational overhead.
- If your primary focus is Time-Dependent Flow: The reduction in calculation load makes SCRS the superior choice for handling the heavy demands of 3D unsteady simulations.
The SCRS model proves that in large-scale engineering simulations, intelligent simplification often yields the most practical and efficient path to accurate results.
Summary Table:
| Feature | SCRS Model Approach | Traditional Detailed Kinetics |
|---|---|---|
| Component Count | 3 (Fuel, Oxidant, Products) | Dozens to Hundreds of Species |
| Calculation Type | Parameter-based Mixing | Complex Differential Equations |
| Computational Speed | High (Ideal for 3D Unsteady) | Low (Hardware Intensive) |
| Primary Output | Temperature & Heat Release | Intermediate Radicals & Trace Species |
| Best For | Large-scale Thermal Profiling | Detailed Chemical Pollutant Tracking |
Optimize Your Thermal Systems with KINTEK
Precision in simulation deserves precision in hardware. KINTEK provides industry-leading thermal solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all designed to meet the rigorous demands of modern lab and industrial research. Backed by expert R&D and manufacturing, our furnaces are fully customizable to your unique simulation and production needs.
Ready to elevate your lab’s high-temperature capabilities? Contact KINTEK today to discuss how our customizable systems can bring your numerical simulations to life.
Visual Guide
References
- O. I. Varfolomeeva, D. A. Khvorenkov. Development of a universal model for numerical analysis of firebox processes in heat-generating plants. DOI: 10.30724/1998-9903-2025-27-6-171-186
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the necessity of carbon coating for silicon anodes? Engineering Durability for High-Performance Batteries
- What role does a forced air drying oven play in the preparation of zinc oxide nanoparticles? Prevent Agglomeration
- Why is high temperature control precision essential for SiC/SiC composites? Master Microstructural Engineering
- What factors influence the time and temperature of the annealing process? Optimize Your Heat Treatment for Better Results
- Why is a vacuum sealing process necessary for the synthesis of TaAs2 single crystals? Ensuring Purity in CVT Method
- How does substrate preheating equipment affect the formation and distribution of the Laves phase in Inconel 718?
- Why is it necessary for each precursor source tube in a multi-source VTD to have an independent MFC? Precision Control
- What are the core technical advantages of a flash sintering system? Elevate KNN Ceramic Manufacturing Performance



















