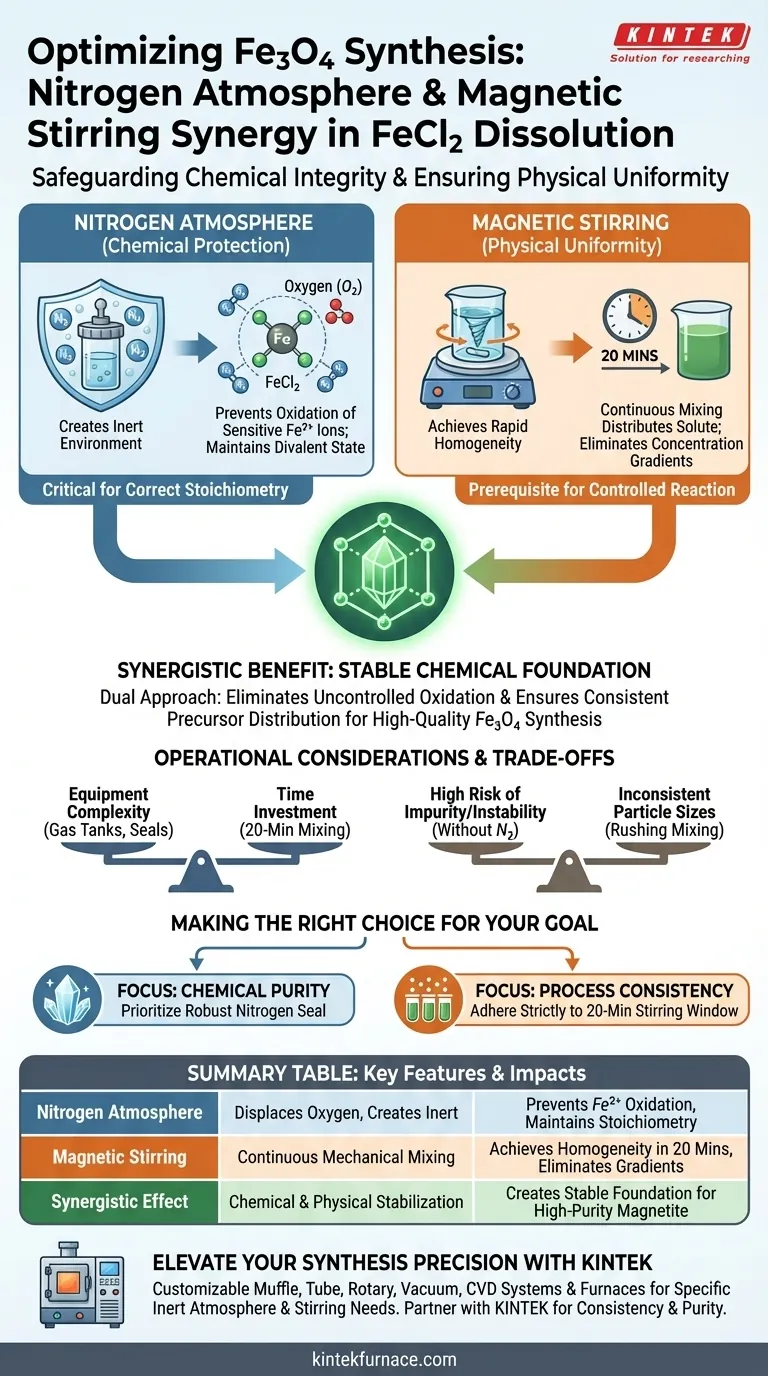
The synergistic combination of a nitrogen atmosphere and magnetic stirring safeguards chemical integrity while ensuring physical uniformity during the dissolution stage. Nitrogen acts as a protective barrier to prevent the oxidation of sensitive ferrous ions, while magnetic stirring guarantees the solution reaches complete homogeneity within 20 minutes.
By simultaneously eliminating uncontrolled oxidation and ensuring consistent precursor distribution, this dual approach creates the stable chemical foundation required for high-quality $Fe_3O_4$ synthesis.

The Role of the Nitrogen Atmosphere
Creating an Inert Environment
The primary function of introducing nitrogen gas is to displace atmospheric oxygen within the reaction vessel.
This creates an inert protective atmosphere specifically designed to shield the solution from the surrounding air.
Protecting Divalent Iron
Ferrous chloride ($FeCl_2$) contains divalent iron ions ($Fe^{2+}$), which are highly susceptible to oxidation.
Without the nitrogen shield, these ions would react with oxygen to form unwanted ferric species ($Fe^{3+}$) before the intended reaction begins.
Maintaining the iron in its divalent state is critical for the correct stoichiometry in the subsequent synthesis steps.
The Impact of Magnetic Stirring
Achieving Rapid Homogeneity
Continuous mixing provided by a magnetic stirrer actively distributes the solute throughout the solvent.
According to established protocols, this mechanical action allows the solution to reach a high degree of homogeneity in approximately 20 minutes.
Establishing Reaction Stability
A uniform solution is the prerequisite for a controlled reaction.
By eliminating concentration gradients, magnetic stirring ensures that when ferric ions are eventually introduced, the reaction occurs consistently throughout the volume.
Operational Considerations and Trade-offs
Equipment Complexity vs. Purity
Implementing a nitrogen purge system adds a layer of complexity to the experimental setup compared to open-air mixing.
It requires gas tanks, regulators, and a sealed reaction vessel, which increases the initial preparation time.
However, omitting this step creates a high risk of uncontrolled oxidation, rendering the final $Fe_3O_4$ product chemically impure or unstable.
Time Investment
The 20-minute mixing period is a necessary investment for consistency.
Rushing this stage or utilizing inadequate mixing methods can lead to incomplete dissolution.
This results in localized reactions and inconsistent particle sizes in the final material.
Making the Right Choice for Your Goal
To maximize the quality of your magnetite synthesis, align your process controls with your purity requirements.
- If your primary focus is Chemical Purity: Prioritize a robust nitrogen seal to strictly maintain the ferrous state of the iron ions, preventing early oxidation.
- If your primary focus is Process Consistency: Adhere strictly to the 20-minute magnetic stirring window to ensure the precursor solution is perfectly homogeneous before proceeding.
A stable, homogeneous precursor solution is the single most important factor in reproducible $Fe_3O_4$ synthesis.
Summary Table:
| Feature | Primary Function | Impact on Fe3O4 Synthesis |
|---|---|---|
| Nitrogen Atmosphere | Displaces oxygen & creates inert environment | Prevents $Fe^{2+}$ oxidation; maintains stoichiometry |
| Magnetic Stirring | Continuous mechanical mixing | Achieves homogeneity in 20 mins; eliminates gradients |
| Synergistic Effect | Chemical & physical stabilization | Creates a stable foundation for high-purity magnetite |
Elevate Your Synthesis Precision with KINTEK
High-quality $Fe_3O_4$ synthesis demands absolute control over environmental variables. At KINTEK, we understand that chemical integrity starts at the dissolution stage. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to support your specific inert atmosphere and stirring requirements.
Don't let uncontrolled oxidation compromise your research. Partner with KINTEK to secure the consistency and purity your laboratory deserves. Contact us today to discuss your custom furnace solution!
Visual Guide
References
- Yingtao Sun, Jianfeng Zhou. Developing and characterizing magnetic nanocomposites for effective metal ion removal in wastewater treatment. DOI: 10.46690/capi.2025.08.03
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does a constant temperature drying oven facilitate solvent removal? Optimize Perovskite Nanocrystal Synthesis
- What is the purpose of setting an industrial drying oven to 70°C for sludge? Preserve Volatiles & Optimize Pre-treatment
- What is the purpose of bottom-entry argon injection? Enhance Lithium-ion Battery Safety & Purge Efficiency
- What is the importance of defining accurate heat transfer coefficients for slag? Master Thermal Stress Prediction
- What role does a high-pressure reactor play in the production of hydrochar? Optimize Biomass Carbonization
- What role does a high-temperature furnace play in APTO for Vanadium to VO2? Precision Phase Transformation Explained
- Why must ultra-high purity argon be continuously supplied for Aluminum-Silicon alloys? Ensure Viscosity Data Accuracy
- How does a heated substrate platform mitigate the coffee ring effect? Enhance Ag2Se Printing Precision



















