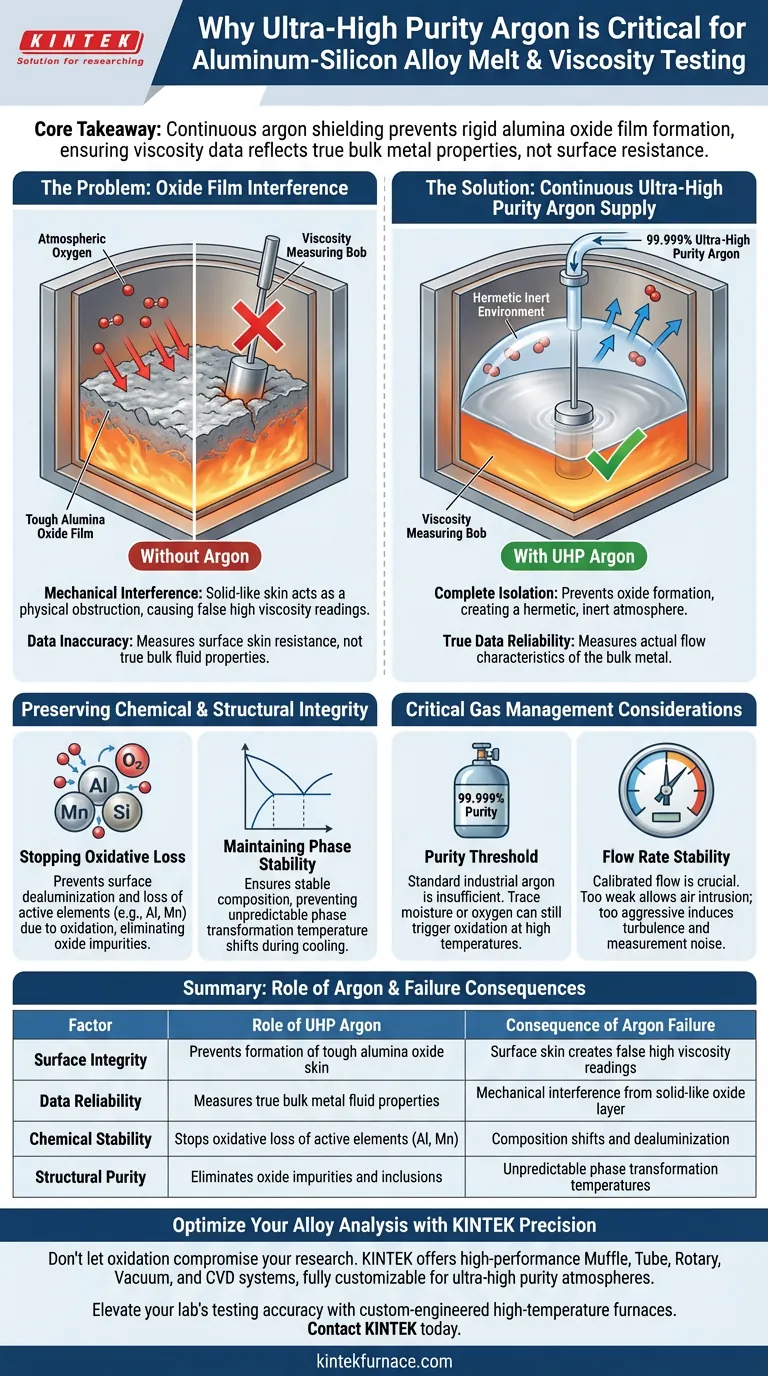
The continuous supply of ultra-high purity argon is strictly required to create a hermetic, inert environment that completely isolates molten aluminum from atmospheric oxygen. Without this protection, the formation of a tough oxide film on the metal's surface physically interferes with measurement instruments, resulting in erroneous viscosity data that reflects the resistance of the surface skin rather than the actual fluid properties of the alloy.
Core Takeaway The primary function of argon shielding is to prevent the formation of a rigid alumina oxide film, which possesses extremely high apparent viscosity. If this film forms, it generates false resistance during testing, masking the true flow characteristics of the bulk metal and rendering the collected data invalid.
The Impact on Viscosity Data Accuracy
Preventing Mechanical Interference
Aluminum is highly reactive with oxygen, particularly at melting temperatures.
Without an inert barrier, a strong alumina oxide film forms almost instantly on the surface of the melt.
This film is not a liquid; it is a tough, solid-like skin that acts as a physical obstruction.
Isolating Bulk Behavior vs. Surface Effects
Viscosity testing measures the internal friction or resistance of a fluid to flow.
When an oxide film is present, the measurement device detects the high mechanical resistance of the surface film rather than the liquid metal beneath it.
This results in data showing an artificially high viscosity, failing to represent the true rheological properties of the bulk metal.
Preserving Chemical and Structural Integrity
Stopping Oxidative Loss
Beyond physical interference, oxygen exposure chemically alters the alloy.
Active elements within the alloy, such as aluminum and manganese, are easily oxidized and effectively "lost" from the matrix.
Ultra-high purity argon (99.999%) prevents this surface dealuminization and the introduction of oxide impurities.
Maintaining Phase Stability
The oxidative loss of alloy components changes the chemical composition of the remaining liquid.
This shift in composition can alter phase transformation temperatures, leading to unpredictable behavior during cooling and solidification.
Argon shielding ensures the alloy's composition remains stable, guaranteeing that test results correlate with the material's intended functional performance.
Critical Considerations in Gas Management
The Purity Threshold
Using standard industrial argon is often insufficient for high-precision testing.
Trace amounts of moisture or oxygen in lower-grade gas can still trigger oxidation at elevated temperatures.
You must utilize ultra-high purity argon to ensure the complete exclusion of contaminants.
Flow Rate Stability
While the supply must be continuous, the flow rate must be carefully calibrated.
A flow that is too weak may allow atmospheric air to diffuse into the furnace chamber.
However, a flow that is too aggressive can induce turbulence in the melt, which introduces physical noise into the viscosity measurement.
Ensuring Data Reliability
To achieve scientifically valid viscosity measurements for Aluminum-Silicon alloys, apply the following protocols:
- If your primary focus is Rheological Accuracy: Ensure the argon supply is active before heating begins to prevent the initial formation of any oxide skin.
- If your primary focus is Microstructural Analysis: Maintain the inert atmosphere through the solid solution treatment stage to prevent surface dealuminization and impurity intrusion.
True data accuracy requires measuring the metal, not the oxide skin that creates it.
Summary Table:
| Factor | Role of Ultra-High Purity Argon | Consequence of Argon Failure |
|---|---|---|
| Surface Integrity | Prevents formation of tough alumina oxide skin | Surface skin creates false high viscosity readings |
| Data Reliability | Measures true bulk metal fluid properties | Mechanical interference from solid-like oxide layer |
| Chemical Stability | Stops oxidative loss of active elements (Al, Mn) | Composition shifts and dealuminization |
| Structural Purity | Eliminates oxide impurities and inclusions | Unpredictable phase transformation temperatures |
Optimize Your Alloy Analysis with KINTEK Precision
Don't let surface oxidation compromise your research data. At KINTEK, we understand that accurate rheological and microstructural analysis starts with a controlled environment. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to maintain the ultra-high purity atmospheres your high-temperature lab work demands.
Ready to elevate your lab's testing accuracy? Contact us today to discover how our custom-engineered high-temperature furnaces can provide the stable, inert environment your Aluminum-Silicon alloy testing requires.
Visual Guide
References
- Antonia P. Betzou, Prakash Srirangam. Effect of Melt Superheat and Shear Rate on Viscosity of Aluminium–Silicon Alloys. DOI: 10.1007/s11663-025-03626-4
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the significance of pre-equilibrating samples in silicate studies? Maximize Experimental Efficiency
- What role does an arc-imaging furnace play in NaMgPO4:Eu synthesis? Rapid Phase Discovery & Olivine Isolation
- What is the purpose of the annealing process in OLED preparation? Optimize Film Stability and Device Efficiency
- Why is a low-temperature annealing treatment necessary for porous gold microspheres? Ensure Structural Integrity
- What are the advantages of HDH niobium powder in Ti-Nb alloys? Optimize Costs and Microstructure
- What role does a laboratory drying oven play in catalyst supports? Ensure Structural Integrity & High Dispersion
- How does oxygen-enhanced alkaline thermal treatment benefit high-purity cellulose pulp? Achieve Superior Fiber Yield
- How does the temperature of the annealing process specifically influence the luminescence properties of ZnSe? Guide



















