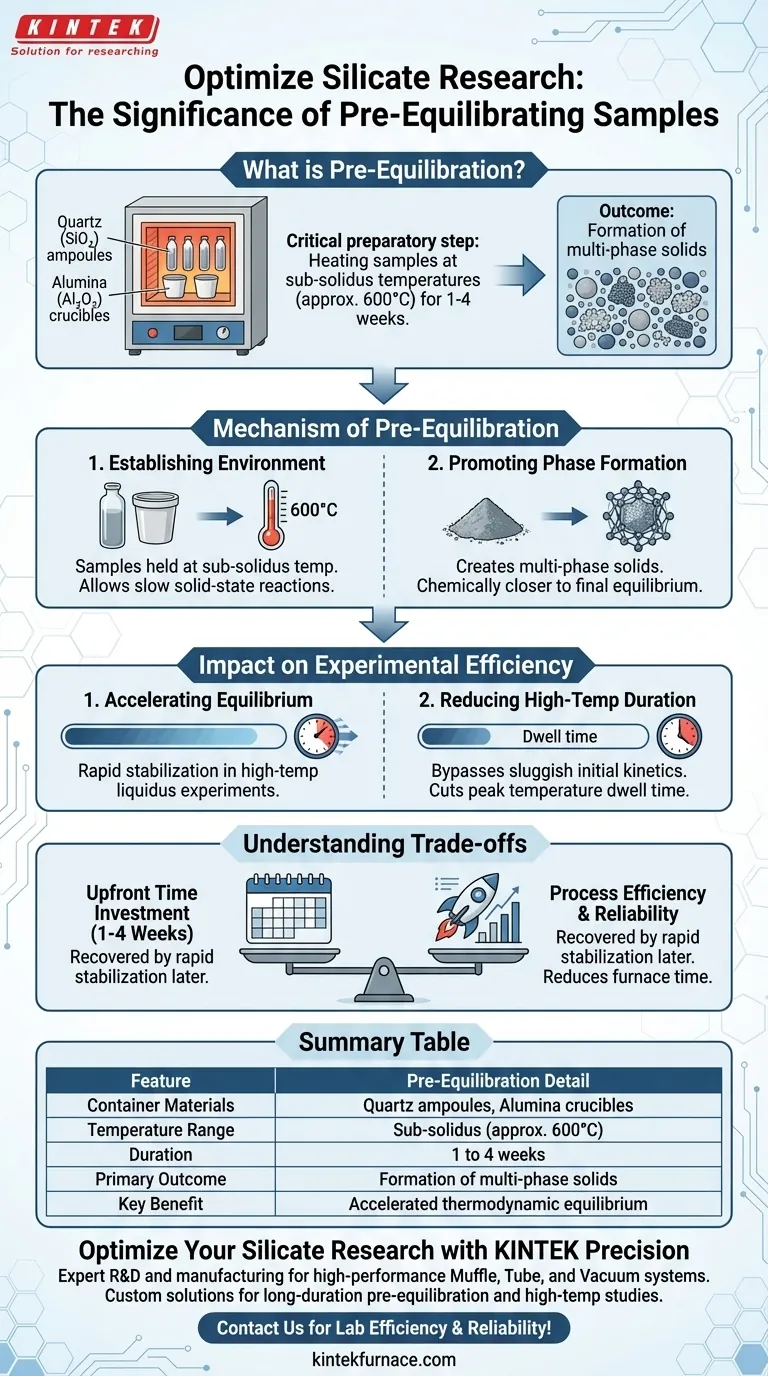
Pre-equilibrating samples serves as a critical preparatory step that optimizes the efficiency of high-temperature silicate experiments. By heating samples in quartz (SiO2) ampoules or alumina (Al2O3) crucibles at sub-solidus temperatures (approximately 600°C) for a duration of 1 to 4 weeks, you effectively "prime" the material for subsequent studies. This process is essential for ensuring that later high-temperature experiments run smoothly and yield reliable results.
Pre-equilibration facilitates the formation of multi-phase solids before the sample is subjected to higher temperatures. This upfront investment significantly reduces the time required to reach thermodynamic equilibrium in later liquidus experiments, thereby increasing overall experimental efficiency.

The Mechanism of Pre-Equilibration
Establishing the Environment
The process begins by placing specific sample compositions into robust containers, specifically quartz ampoules or alumina crucibles.
These samples are then held at sub-solidus temperatures, generally around 600°C.
This environment allows the materials to react slowly without melting, a state known as solid-state reaction.
Promoting Phase Formation
The primary chemical objective during this 1-4 week period is the formation of multi-phase solids.
Rather than starting a high-temperature experiment with raw, unreacted powders, you create a complex solid mixture.
This ensures the starting material is chemically closer to the final equilibrium state needed for later studies.
The Impact on Experimental Efficiency
Accelerating Thermodynamic Equilibrium
The most significant benefit of this method is time management during critical experimental phases.
When you eventually subject these pre-equilibrated samples to high-temperature liquidus experiments, they reach thermodynamic equilibrium much faster.
Reducing High-Temperature Duration
Achieving equilibrium in silicate melts can be notoriously slow if starting from scratch.
By pre-equilibrating, you bypass the initial sluggish reaction kinetics associated with raw starting materials.
This drastically cuts down the dwell time required at peak temperatures to get valid data.
Understanding the Trade-offs
Upfront Time vs. Process Efficiency
The most obvious implication of this method is the initial time investment.
You must allocate 1 to 4 weeks for preparation before the "real" high-temperature experiment begins.
However, this "lost" time is generally recovered by the rapid stabilization of the sample during the more complex liquidus phase.
Resource Allocation
Using quartz or alumina containers implies a need for materials compatible with these specific thermal conditions.
While this adds a layer of preparation, it prevents the inefficiency of running high-temperature furnaces for extended periods solely to wait for equilibrium.
Making the Right Choice for Your Goal
To determine if this protocol fits your experimental design, consider your constraints regarding time and accuracy.
- If your primary focus is total project efficiency: Commit to the 1-4 week pre-equilibration phase to minimize the expensive and time-consuming duration of high-temperature runs.
- If your primary focus is experimental reliability: Use this method to ensure your starting materials are chemically homogeneous multi-phase solids, reducing the risk of non-equilibrium results.
By investing time in sub-solidus pre-equilibration, you trade upfront patience for reliable, high-efficiency data collection later.
Summary Table:
| Feature | Pre-Equilibration Detail |
|---|---|
| Container Materials | Quartz (SiO2) ampoules or Alumina (Al2O3) crucibles |
| Temperature Range | Sub-solidus (approx. 600°C) |
| Duration | 1 to 4 weeks |
| Primary Outcome | Formation of multi-phase solids |
| Key Benefit | Accelerated thermodynamic equilibrium in liquidus experiments |
Optimize Your Silicate Research with KINTEK Precision
High-accuracy silicate studies demand reliable thermal environments. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, and Vacuum systems specifically designed for long-duration sub-solidus pre-equilibration and high-temperature liquidus studies.
Whether you need standard alumina crucibles or fully customizable furnace systems for unique material constraints, our team is ready to support your lab's specific needs. Contact us today to enhance your lab's efficiency and data reliability!
Visual Guide
References
- Georgii Khartcyzov, Evgueni Jak. Integrated Experimental and Thermodynamic Modelling Study of Phase Equilibria in the PbO-AlO1.5-SiO2 System in Air. DOI: 10.1007/s12540-024-01878-4
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- How does a high-precision reaction system aid methane CLR research? Unlock Advanced Syngas Insights
- What is the purpose of performing high-temperature thermal treatment for BSnO thin films? Boost Device Sensitivity
- What is the role of MgO powder in Nickel-Aluminum VCS? Achieve Precise Thermal Control & Powder Quality
- What is the impact of using a vacuum drying oven on CDI electrodes? Optimize Stability and Conductivity
- Why is a vacuum freeze dryer used for Vivianite? Optimize Your LFP Synthesis with Superior Precursor Integrity
- What is the function of an industrial drying oven in EFB fiber pretreatment? Optimize Biochar Yield & Quality
- What is the significance of preheating UHPC molds? Ensure Safety & Longevity with High-Temp Furnaces
- Why is a steam generator and programmable furnace needed for emission aging? Replicate Real Hydrothermal Environments



















