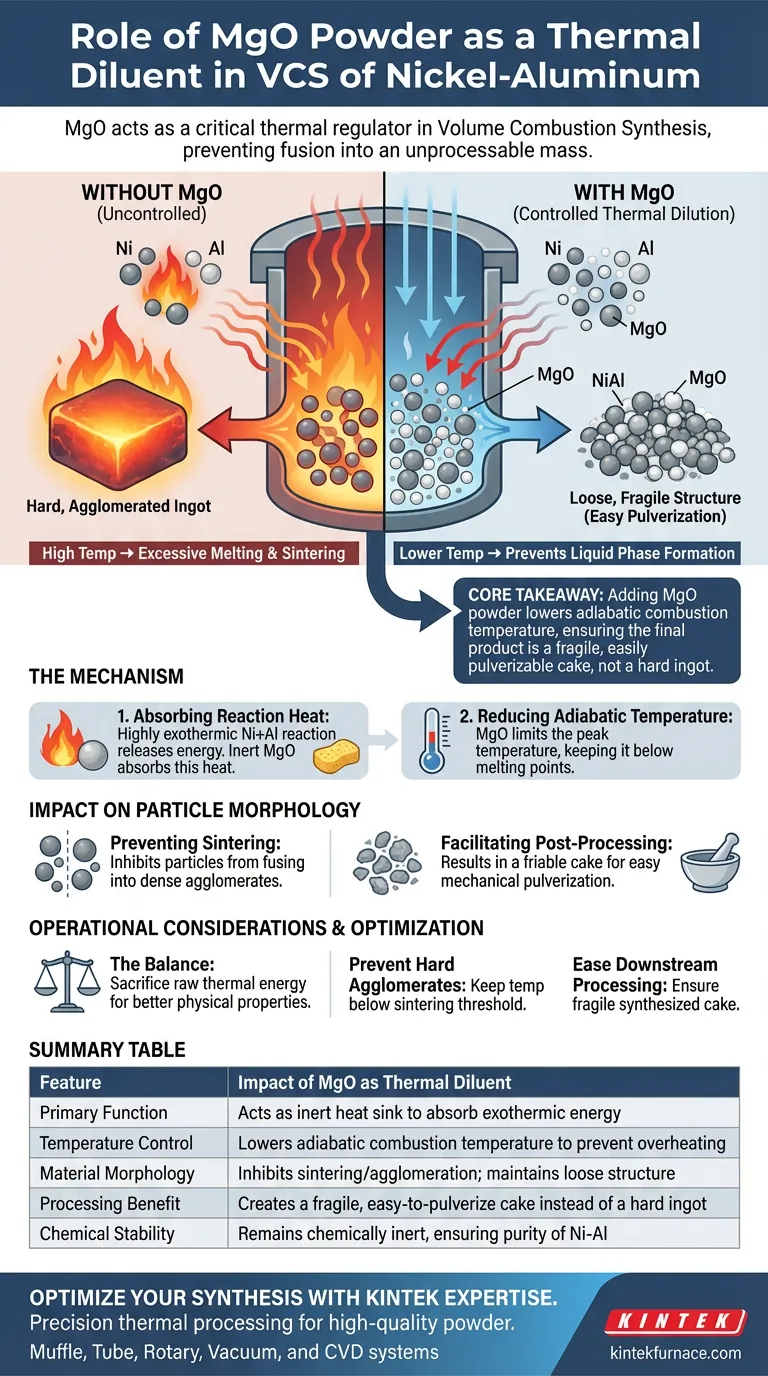
In the Volume Combustion Synthesis (VCS) of Nickel-Aluminum alloys, MgO powder serves as a critical thermal regulator that maintains control over the reaction environment. It acts primarily as a heat sink, absorbing excess energy to prevent the synthesized material from fusing into an unprocessable solid mass.
Core Takeaway Adding MgO powder lowers the adiabatic combustion temperature of the reaction, preventing excessive melting and sintering. This ensures the final Nickel-Aluminum product remains a loose, fragile structure that is easy to pulverize, rather than a hard, agglomerated ingot.

The Mechanism of Thermal Dilution
Absorbing Reaction Heat
The reaction between Nickel and Aluminum is highly exothermic, releasing significant amounts of energy. MgO, being a high-melting-point ceramic, is introduced to the mixture as a chemically inert "diluent."
Its primary function is to absorb a portion of the heat generated during the synthesis. Because it does not react with the metal powders, it effectively dampens the thermal intensity of the system.
Reducing Adiabatic Temperature
By absorbing this heat, the MgO reduces the adiabatic combustion temperature (the maximum theoretical temperature the reaction reaches).
Keeping this temperature in check is vital. If the temperature rises unchecked, it can exceed the melting points of the constituents, leading to a loss of microstructural control.
Impact on Particle Morphology
Preventing Sintering and Agglomeration
High temperatures during VCS typically cause the newly formed NiAl particles to melt and bond together, a process known as sintering.
By lowering the reaction temperature, MgO prevents this excessive liquid phase formation. This inhibits the particles from fusing into large, dense agglomerates.
Facilitating Post-Processing
The physical state of the final product is determined by the peak temperature reached during synthesis.
Because MgO limits melting, the resulting product is a loose and fragile cake. This friability is a major operational advantage, as it allows for easy pulverization into the final powder form without requiring heavy-duty crushing equipment.
Operational Considerations
The Necessity of Balance
While the primary reference focuses on the benefits, it is important to view the diluent as a control lever.
The addition of MgO is a deliberate trade-off. You are introducing an inert material to sacrifice raw thermal energy in exchange for better physical properties and easier handling of the final product.
Optimizing Your Synthesis Strategy
To achieve the best results in Nickel-Aluminum VCS, consider how the quantity of diluent aligns with your production goals.
- If your primary focus is preventing hard agglomerates: Use MgO to keep the combustion temperature below the threshold where rapid sintering occurs.
- If your primary focus is easing downstream processing: Leverage the diluent effect to ensure the synthesized cake is fragile enough for simple mechanical pulverization.
By using MgO to modulate the thermal energy of the reaction, you ensure a process that is both safe and capable of yielding high-quality, manageable powder.
Summary Table:
| Feature | Impact of MgO as Thermal Diluent |
|---|---|
| Primary Function | Acts as an inert heat sink to absorb exothermic reaction energy |
| Temperature Control | Lowers adiabatic combustion temperature to prevent overheating |
| Material Morphology | Inhibits sintering/agglomeration; maintains loose structure |
| Processing Benefit | Creates a fragile, easy-to-pulverize cake instead of a hard ingot |
| Chemical Stability | Remains chemically inert, ensuring purity of Ni-Al intermetallics |
Optimize Your Synthesis with KINTEK Expertise
Precision in thermal processing is the difference between a fused mass and a high-quality powder. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which are fully customizable for your unique Volume Combustion Synthesis needs.
Whether you are synthesizing intermetallics or advanced ceramics, our lab high-temp furnaces provide the thermal stability required to manage complex exothermic reactions. Contact us today to discuss how our specialized equipment can enhance your material science workflows!
Visual Guide
References
- Gülizar Sarıyer, H. Erdem Çamurlu. Production and Characterization of Ni0.50 Al0.50 and Ni0.55 Al0.45 Powders by Volume Combustion Synthesis. DOI: 10.17776/csj.1280582
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What role does an industrial box-type resistance furnace play in phosphor conversion? Powering Material Synthesis
- Why is a Rapid Thermal Processing (RTP) furnace necessary for diode fabrication? Achieve Stable Ohmic Contacts
- What is the role of high-purity argon gas in ultrafine magnesium powder production? Control Particle Size & Purity
- How does the aluminum precursor coating process modify high-purity quartz? Enhancing Thermal Stability and Viscosity
- What is the primary function of a laboratory electric drying oven in ACBP production? Ensure Precise Pre-treatment
- What are the advantages of Spark Plasma Sintering (SPS)? Enhance Thermoelectric Performance in Copper Sulfide
- Why is it necessary to grind iron concentrate to 5-10 microns? Optimize Your Iron Ore Reduction Kinetics
- Why is HR-TEM used after high-temperature heat treatment? Visualize structural evolution and material integrity.



















