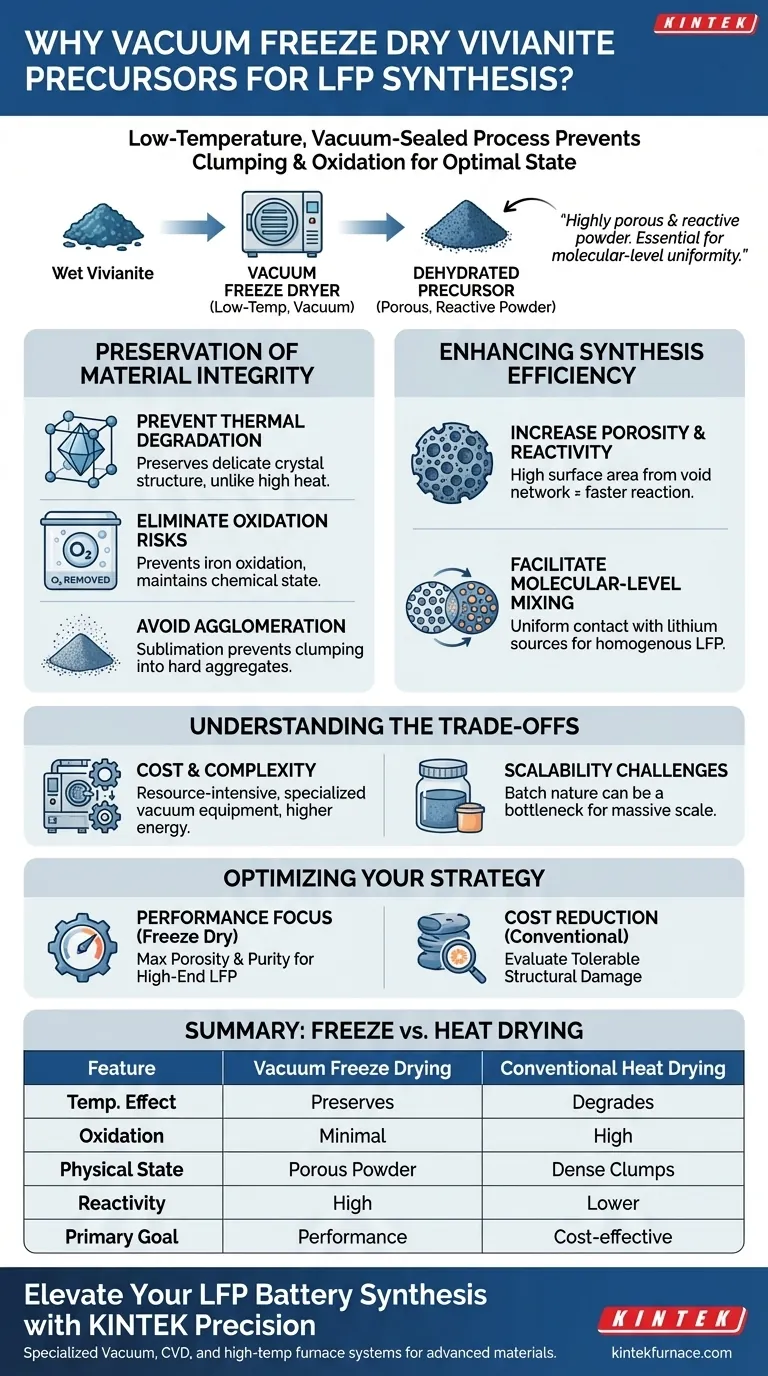
A vacuum freeze dryer is employed to dehydrate wet Vivianite without subjecting it to the destructive effects of high heat. This low-temperature, vacuum-sealed process prevents the material from clumping, oxidizing, or suffering structural damage, ensuring the precursor remains in an optimal state for chemical synthesis.
By avoiding thermal stress, freeze drying creates a highly porous and reactive powder. This physical state is essential for achieving the molecular-level uniformity required when mixing with lithium sources for Lithium Iron Phosphate (LFP) production.

The Preservation of Material Integrity
Preventing Thermal Degradation
Conventional drying methods rely on heat to evaporate moisture. High temperatures can alter the delicate crystal structure of Vivianite.
Freeze drying operates in a low-temperature environment. This preserves the original crystalline framework of the precursor, ensuring it retains the specific properties needed for successful conversion into LFP.
Eliminating Oxidation Risks
Iron-based compounds like Vivianite are highly susceptible to oxidation when exposed to air and heat. Oxidation changes the valence state of the iron, which is detrimental to battery performance.
The vacuum environment of a freeze dryer removes oxygen during the drying process. This ensures the iron remains in its intended chemical state, preventing impurities from forming before the synthesis even begins.
avoiding Agglomeration
Wet precipitates tend to clump together into hard aggregates when dried in an oven. These dense clumps are difficult to break down later.
Freeze drying sublimates the ice directly into vapor. This leaves the solid particles undisturbed, preventing them from fusing into hard masses and resulting in a fine, loose powder.
Enhancing Synthesis Efficiency
Increasing Porosity and Reactivity
Because the water leaves the material via sublimation, it leaves behind a network of voids. This results in a powder with high porosity.
This increased surface area translates directly to higher chemical reactivity. The precursor is more "available" to react, making the subsequent synthesis process more efficient.
Facilitating Molecular-Level Mixing
The ultimate goal is to mix the Vivianite with a lithium source. A dense, clumpy precursor results in uneven mixing.
The freeze-dried powder's high porosity allows for molecular-level uniform contact with lithium sources. During milling and grinding, the lithium can penetrate the Vivianite structure more effectively, leading to a homogenous final product.
Understanding the Trade-offs
Cost and Complexity
While freeze drying yields a superior precursor, it is significantly more resource-intensive than oven drying.
The process requires specialized vacuum equipment and consumes more energy to maintain low temperatures and vacuum pressure. It also typically takes longer to complete a drying cycle than thermal methods.
Scalability Challenges
For massive industrial scales, the batch nature of freeze drying can be a bottleneck. Manufacturers must weigh the improved electrochemical performance against the lower throughput and higher operational costs.
Optimizing Your Synthesis Strategy
To determine if freeze drying is the right approach for your specific application, consider your performance targets.
- If your primary focus is electrochemical performance: Prioritize freeze drying to maximize porosity, reactivity, and purity for a high-end LFP battery.
- If your primary focus is cost reduction: Evaluate if the potential structural damage from conventional drying is within acceptable tolerance limits for your specific grade of material.
Ultimately, the choice of drying method dictates the homogeneity of your precursors, which is the single biggest predictor of final battery consistency.
Summary Table:
| Feature | Vacuum Freeze Drying | Conventional Heat Drying |
|---|---|---|
| Temperature Effect | Preserves crystal structure; no thermal stress | Risk of structural degradation and sintering |
| Oxidation Risk | Minimal (vacuum environment) | High (exposure to air and heat) |
| Physical State | High porosity, loose fine powder | Dense clumps and hard agglomerates |
| Reactivity | High surface area; molecular-level mixing | Lower reactivity; difficult to homogenize |
| Primary Goal | Maximum battery performance & purity | Cost-effective high-volume production |
Elevate Your LFP Battery Synthesis with KINTEK Precision
Maximize the electrochemical performance of your lithium iron phosphate production by ensuring perfect precursor homogeneity. Backed by expert R&D and manufacturing, KINTEK offers specialized Vacuum, CVD, and high-temp furnace systems, including customizable solutions for advanced material synthesis. Whether you are processing Vivianite or developing next-generation cathodes, our equipment provides the thermal and atmospheric control your lab requires.
Ready to achieve molecular-level uniformity in your precursors? Contact KINTEK today to discuss your custom lab equipment needs!
Visual Guide
References
- Tengshu Chen, Liyao Chen. Research on the synthesis of lithium iron phosphate using vivianite prepared from municipal sludge. DOI: 10.1038/s41598-025-16378-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What are the advantages of Flash Lamp Annealing (FLA)? Unlock High-Performance Films on Heat-Sensitive Substrates
- Why is a graphite furnace better than a flame in AAS? Unlock Trace-Level Detection for Your Lab
- Why is a furnace with programmed temperature control required for catalyst regeneration? Ensure Catalyst Stability
- What roles does a laboratory oven play in biochar production? Enhance Efficiency and Accuracy in Thermal Processing
- How does a constant temperature heating device influence battery performance? Enhance Lithium Dendrite Research Accuracy
- Why is the purity of oxide precursors critical for ZnO-doped CuO? Ensure High Photocatalytic Performance
- How does a high-power microwave reactor facilitate the thermal treatment of zinc clinker? Rapid Phase Transformation
- What is the primary purpose of using nano-magnesium oxide as a template? Optimize Sulfur-Doped Porous Carbon Synthesis



















