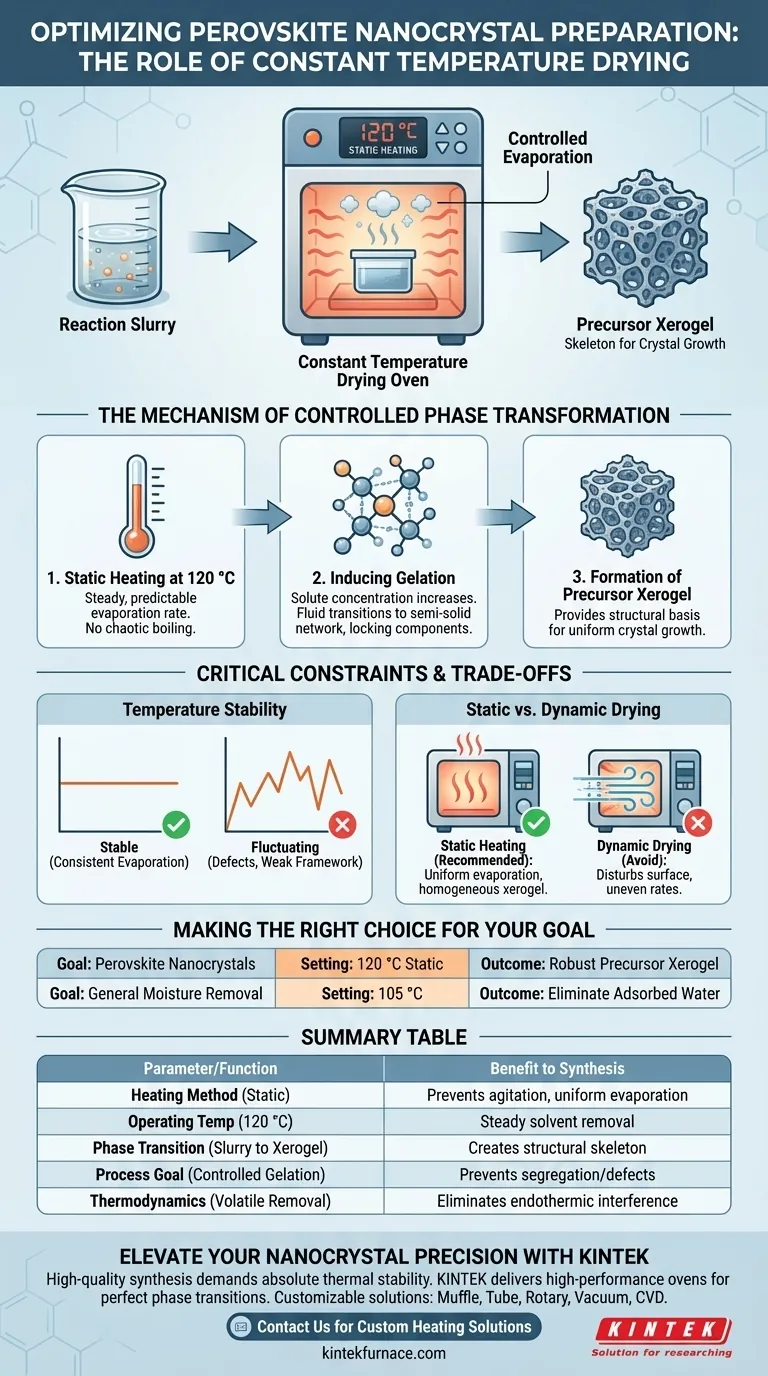
In perovskite nanocrystal preparation, a constant temperature drying oven facilitates solvent removal by subjecting the reaction slurry to long-term static heating, typically at 120 °C. This precise thermal environment drives controlled evaporation, transforming the liquid mixture into a stable precursor xerogel.
The oven’s function extends beyond simple drying; it orchestrates a phase transition from slurry to gel. By ensuring smooth solvent removal, it creates the essential structural foundation required for high-quality crystal growth.

The Mechanism of Controlled Phase Transformation
Static Heating at 120 °C
The process begins by placing the reaction slurry into the oven. Unlike dynamic heating methods that might agitate the mixture, the oven provides static heating.
The standard operating temperature is maintained at 120 °C. This specific thermal setting is calibrated to ensure the solvent evaporates at a steady, predictable rate rather than boiling off chaotically.
Inducing Gelation
As the solvent evaporates under these controlled conditions, the concentration of the solute increases. This gradual change triggers gelation.
The mixture transitions from a fluid state into a semi-solid network. This step is critical because it locks the chemical components into place, preventing them from segregating or precipitating unevenly.
Formation of the Precursor Xerogel
The ultimate output of this drying phase is a precursor xerogel.
This solid structure acts as the "skeleton" for the final material. It provides the structural basis necessary for the subsequent steps of crystal growth, ensuring the final nanocrystals have a uniform framework.
Understanding the Trade-offs and Critical Constraints
The Importance of Temperature Stability
Precision is non-negotiable. If the temperature fluctuates significantly, the rate of solvent removal becomes inconsistent.
Inconsistent evaporation can disrupt the gelation process. This often leads to structural defects or a weak precursor framework that cannot support proper crystal formation.
Static vs. Dynamic Drying
It is important to distinguish this process from high-temperature blast drying used for other materials (such as stabilizing composite precipitates on substrates).
For perovskite precursors, the primary reference emphasizes static heating. Introducing strong air currents (blast drying) could disturb the slurry surface or cause uneven evaporation rates across the sample, potentially compromising the homogeneity of the resulting xerogel.
Managing Endothermic Effects
While the primary goal is xerogel formation, thorough solvent removal also serves a thermodynamic purpose.
Drawing from general drying principles, removing volatile components prevents unwanted endothermic effects during later high-temperature processing. Residual solvents can absorb heat unexpectedly, destabilizing the thermal conditions required for the final reaction.
Making the Right Choice for Your Goal
To ensure the success of your synthesis, match your drying strategy to your specific material requirements:
- If your primary focus is Perovskite Nanocrystals: Maintain a static temperature of 120 °C to promote smooth gelation and the formation of a robust precursor xerogel.
- If your primary focus is General Moisture Removal: Set the oven to 105 °C to eliminate physically adsorbed water and prevent thermal interference in subsequent experimental steps.
Success in nanocrystal preparation relies not just on removing the solvent, but on controlling how it is removed to build a perfect structural foundation.
Summary Table:
| Feature | Parameter/Function | Benefit to Perovskite Synthesis |
|---|---|---|
| Heating Method | Long-term Static Heating | Prevents slurry agitation and ensures uniform evaporation |
| Operating Temp | 120 °C | Steady solvent removal without chaotic boiling |
| Phase Transition | Slurry to Xerogel | Creates the structural 'skeleton' for crystal growth |
| Process Goal | Controlled Gelation | Prevents chemical segregation and structural defects |
| Thermodynamics | Volatile Removal | Eliminates endothermic interference in later stages |
Elevate Your Nanocrystal Precision with KINTEK
High-quality perovskite synthesis demands more than just heat—it requires absolute thermal stability and control. KINTEK delivers high-performance constant temperature drying ovens designed to facilitate perfect phase transitions and robust xerogel formation.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for your unique research needs. Ensure the structural integrity of your precursors with our precision engineering.
Ready to optimize your lab's solvent removal process? Contact us today to find your custom heating solution!
Visual Guide
References
- Lebohang Kekana, Ndzondelelo Bingwa. Inorganic SrMo<sub>1–<i>x</i></sub>Ni<sub><i>x</i></sub>O<sub>3</sub><sub>–δ</sub> Perovskite Nanocrystals for Catalytic Reductive Etherification of Biobased Compounds. DOI: 10.1021/acsomega.4c06455
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the purpose of using a forced-air drying oven at 100 °C? Optimize Fe3O4@Fe-AC Composite Synthesis
- What role does active carbon play in CaS:Eu2+ phosphor synthesis? Key to Activating High-Efficiency Luminescence
- In gas-phase aluminizing, how do high-temperature furnaces facilitate the formation of the β-NiAl phase?
- Why use 10% Carbon Monoxide in black liquor pyrolysis? Prevent sodium volatilization for superior char quality.
- How does a constant temperature and humidity curing chamber contribute to alkali-activated material performance?
- Why is vacuum sealing technology essential for K2In2As3 synthesis? Master High-Purity Solid-State Reactions
- How does the lab oven drying process ensure the quality of bimetallic catalysts? Master Pore Stability & Dispersion
- Why is a laboratory electric blast drying oven necessary for determining the water absorption rate of mortar?



















