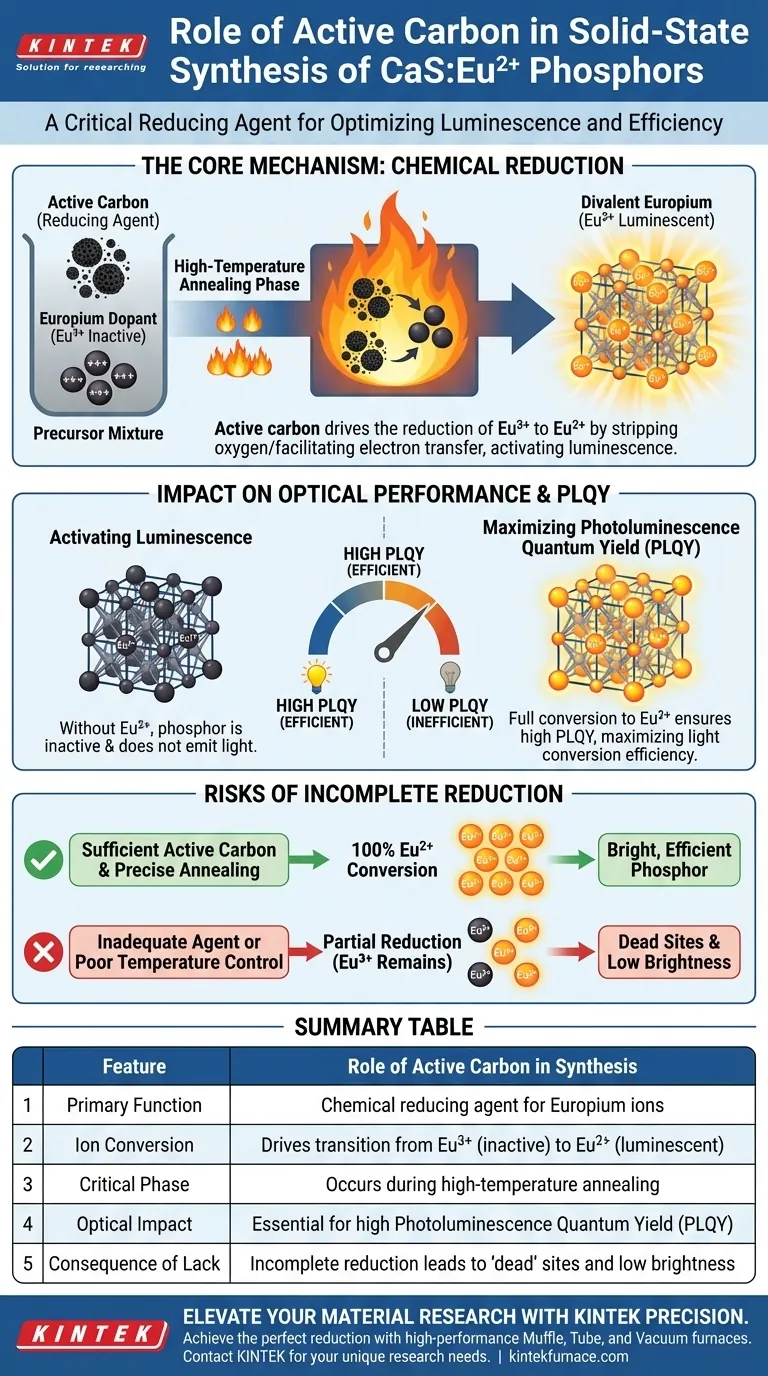
Active carbon functions as a critical reducing agent. In the solid-state synthesis of CaS:Eu2+ phosphors, it is added to the precursor mixture to control the oxidation state of the Europium dopant. Its primary role is to drive the chemical reduction of trivalent Europium (Eu3+) ions into divalent Europium (Eu2+) ions during high-temperature annealing.
The presence of active carbon is the determining factor in activating the luminescent properties of the material. By ensuring the complete reduction of the dopant, it enables the high photoluminescence quantum yield (PLQY) necessary for efficient phosphor performance.

The Mechanism of Reduction
Regulating the Oxidation State
The central challenge in synthesizing CaS:Eu2+ is that Europium naturally exists in a stable trivalent state (Eu3+). However, for the phosphor to be functional, the dopant must be in the divalent state (Eu2+).
Active carbon acts as a chemical lever to force this transition. By introducing it into the precursor mixture, you create a reducing environment that strips oxygen or facilitates electron transfer, converting the inactive Eu3+ into the luminescently active Eu2+.
The Role of High-Temperature Annealing
This chemical reaction is not passive; it requires energy. The reduction process mediated by active carbon occurs specifically during the high-temperature annealing phase.
The heat activates the carbon, allowing it to interact effectively with the Europium ions within the crystal lattice. This ensures that the reduction is thorough and uniform throughout the material.
Impact on Optical Performance
Activating Luminescence
The valence state of the Europium ion dictates the optical behavior of the phosphor. Eu3+ ions do not provide the desired luminescence in this host lattice.
By utilizing active carbon to achieve a full conversion to Eu2+, you unlock the material's ability to emit light. The active carbon is therefore not just an additive; it is the key to "switching on" the phosphor.
Maximizing Photoluminescence Quantum Yield (PLQY)
The ultimate measure of a phosphor's efficiency is its Photoluminescence Quantum Yield (PLQY). This metric represents the efficiency with which the material converts absorbed light into emitted light.
The primary reference indicates a direct correlation between the reduction efficiency and PLQY. Without sufficient active carbon to drive the reduction, the PLQY drops significantly, rendering the phosphor inefficient.
Risks of Incomplete Reduction
The Cost of Insufficient Agent
If the reducing environment is inadequate—due to a lack of active carbon or improper dispersion—a portion of the dopant will remain as Eu3+.
This results in "dead" sites within the phosphor that absorb energy without emitting the desired light, or that emit at incorrect wavelengths.
Process Sensitivity
The synthesis relies heavily on the high-temperature annealing step to facilitate the carbon's reducing action.
If the temperature profile is not maintained correctly, the active carbon may not react fully. This leads to partial reduction, compromising the final brightness and efficiency of the phosphor.
Making the Right Choice for Your Synthesis
To optimize your CaS:Eu2+ phosphor synthesis, consider your specific performance targets:
- If your primary focus is Maximum Brightness (High PLQY): Prioritize the precise stoichiometry of active carbon to ensure there is enough reducing agent to convert 100% of the Eu3+ ions to Eu2+.
- If your primary focus is Process Consistency: Tightly control the high-temperature annealing phase, as this is the specific window where the active carbon performs its critical reduction work.
The effectiveness of your final phosphor is directly constrained by the efficiency of the reduction driven by active carbon.
Summary Table:
| Feature | Role of Active Carbon in Synthesis |
|---|---|
| Primary Function | Chemical reducing agent for Europium ions |
| Ion Conversion | Drives the transition from Eu3+ (inactive) to Eu2+ (luminescent) |
| Critical Phase | Occurs during high-temperature annealing |
| Optical Impact | Essential for high Photoluminescence Quantum Yield (PLQY) |
| Consequence of Lack | Incomplete reduction leads to 'dead' sites and low brightness |
Elevate Your Material Research with KINTEK Precision
Achieving the perfect reduction environment for CaS:Eu2+ phosphors requires precise thermal control. KINTEK provides high-performance, customizable Muffle, Tube, and Vacuum furnaces designed to maintain the exact temperature profiles your synthesis demands.
Backed by expert R&D and manufacturing, our systems ensure consistent high-temperature annealing for advanced material science and lab applications.
Ready to optimize your phosphor performance? Contact KINTEK today to find the ideal furnace for your unique research needs.
Visual Guide
References
- Arzu Coşgun Ergene, Andrey Turshatov. High Photoluminescence Quantum Yield and Tunable Luminescence Lifetimes in the Sub‐Second Range of CaS:Eu<sup>2+</sup> Phosphors for Tracer Based Sorting. DOI: 10.1002/admt.202500353
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
People Also Ask
- What is the effect of 750°C to 950°C on activated carbon? Optimize Pore Structure & Surface Area
- What are the main types of sintering methods for metals, ceramics, and refractory intermetallic compounds powders? Optimize Your Material Processing
- How does an oil circulation heating and cooling system affect HPDC? Optimize Your Die Casting Thermal Control
- What is the role of a precision annealing furnace in the preparation of ZnO or CuO doped phosphate glass?
- What is the technical objective of performing thermal oxidation at 625 °C? Mastering SiOx Tunnel Oxide Precision
- What is the purpose of performing a quenching treatment? Optimize Doped Alkali Halide Crystal Spectral Analysis
- What is the role of a high-energy ball mill in NiWO4/GO preparation? Master High-Performance Composite Synthesis
- Why is a laboratory blast drying oven necessary for preparing Reduced Graphene Oxide precursors? Ensure Powder Quality



















