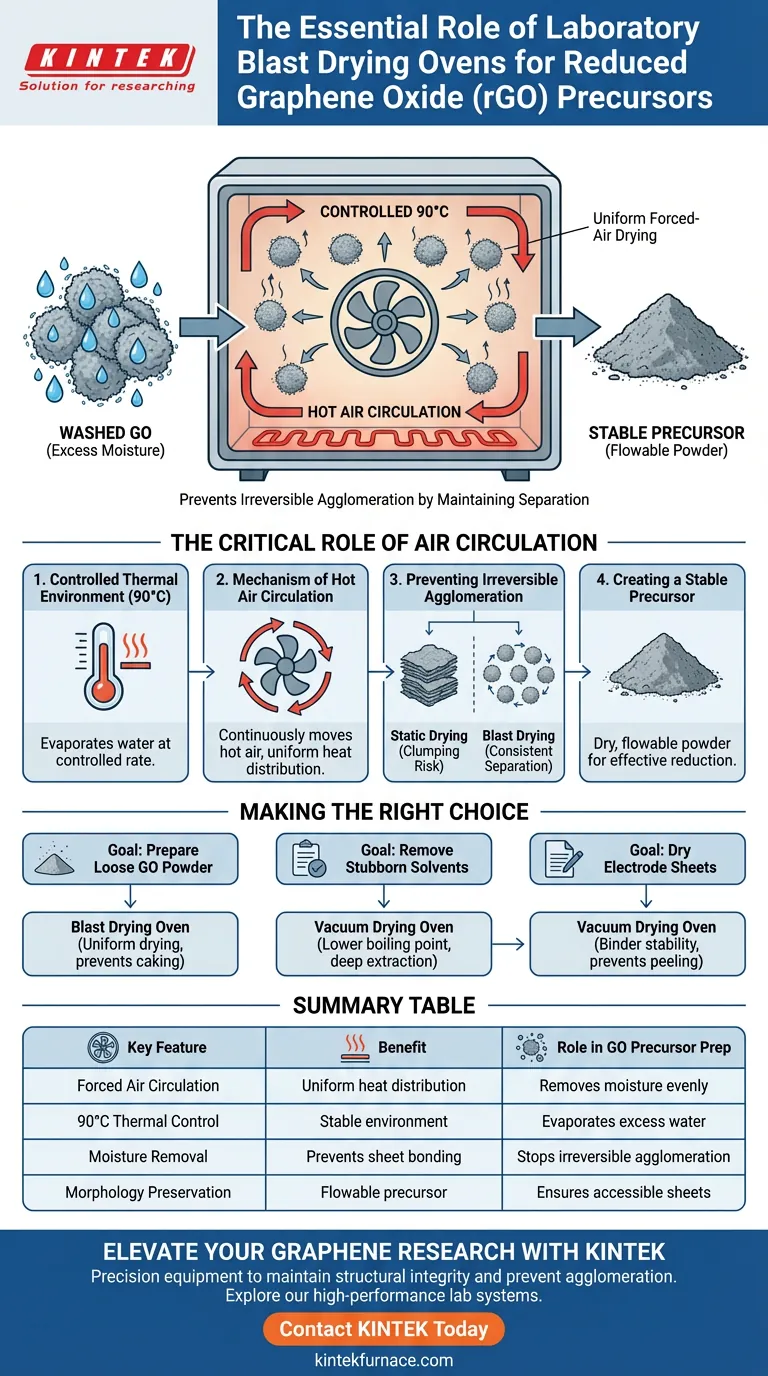
A laboratory blast drying oven is the standard tool for converting washed Graphene Oxide (GO) into a stable precursor powder without compromising its structure. By maintaining a constant temperature, typically around 90 °C, and utilizing continuous hot air circulation, the oven gently removes excess moisture. This controlled environment is essential to prevent the wet GO sheets from sticking together permanently, ensuring the material remains suitable for subsequent chemical or thermal reduction.
The primary function of the blast drying oven is to prevent irreversible agglomeration through uniform, forced-air drying. By preserving the separation of Graphene Oxide sheets during moisture removal, you ensure the precursor powder retains the physical stability and morphology required for high-quality Reduced Graphene Oxide (rGO).

The Critical Role of Air Circulation
Controlled Thermal Environment
After the washing phase, Graphene Oxide contains significant excess moisture.
A blast drying oven creates a stable environment, usually set to 90 °C, to evaporate this water at a controlled rate.
The Mechanism of Hot Air Circulation
The defining feature of a blast oven is its forced air circulation system.
Unlike static drying, this system continuously moves hot air around the sample, ensuring that heat is distributed evenly across the material.
This uniformity is vital for removing free moisture from between particles without creating "hot spots" that could damage the material.
Preventing Irreversible Agglomeration
The most significant risk during the drying of Graphene Oxide is agglomeration.
If GO sheets dry unevenly or too slowly in a static environment, they tend to stack and bond tightly together.
Blast drying mitigates this by drying the material consistently, preventing the formation of hard, irreversible clumps that would be difficult to reduce later.
Creating a Stable Precursor
The result of this process is a dry, loose powder with stable physical morphology.
This "flowable" state allows chemicals or heat to interact with the individual sheets effectively during the next stage: the reduction process to create rGO.
Understanding the Trade-offs
Blast Drying vs. Vacuum Drying
While blast drying is excellent for general powder preparation, it operates at atmospheric pressure.
If your material has deep residual solvents (like methanol) trapped in micropores, a vacuum drying oven might be more effective.
Limitations Regarding Binders
Blast drying relies on heat and air movement, which can be aggressive for certain delicate composites.
For example, when drying electrode sheets, vacuum drying is often preferred to protect binder performance and prevent active material detachment.
However, for the specific goal of preparing raw Graphene Oxide powder, the blast oven remains the superior choice for preventing physical clumping.
Making the Right Choice for Your Goal
To ensure the best results for your specific stage of research, apply the following guidelines:
- If your primary focus is preparing loose GO powder: Use a blast drying oven to ensure uniform drying and prevent the sheets from permanently caking together.
- If your primary focus is removing stubborn solvents from pores: Consider a vacuum drying oven to lower the boiling point and extract residuals without excessive heat.
- If your primary focus is drying finished electrode sheets: Use vacuum drying to ensure binder stability and prevent the peeling of active materials.
By prioritizing airflow and controlled temperature, you protect the structural integrity of your precursor before the reduction process begins.
Summary Table:
| Key Feature | Benefit | Role in GO Precursor Prep |
|---|---|---|
| Forced Air Circulation | Uniform heat distribution | Removes moisture evenly without creating damaging hot spots |
| 90°C Thermal Control | Stable environment | Evaporates excess water at a controlled rate to protect morphology |
| Moisture Removal | Prevents sheet bonding | Stops irreversible agglomeration of Graphene Oxide layers |
| Morphology Preservation | Flowable precursor | Ensures individual sheets remain accessible for the reduction phase |
Elevate Your Graphene Research with KINTEK
Precision is paramount when preparing sensitive precursors like Graphene Oxide. KINTEK provides the high-performance laboratory equipment necessary to maintain structural integrity and prevent material agglomeration. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to meet your unique research requirements.
Ready to optimize your drying and reduction processes?
Contact KINTEK Today to discover how our advanced heating solutions can enhance your material quality and laboratory efficiency.
Visual Guide
References
- Dilek Öztekin, Sena Yaşyerli. Preparation of RGO with Enhanced Electrical Conductivity: Effects of Sequential Reductions of L-Ascorbic Acid and Thermal. DOI: 10.1007/s13369-024-09915-5
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does precise heating rate control affect nitrogen-doped carbon synthesis? Master Thermal Ramp for Quality Materials
- Why must Sm:YAG ceramics undergo air annealing? Restoring Optical Clarity and Restructuring Defects
- Why is pre-sintering of Ga2O3 raw material powder required? Unlock Beta-Phase Stability for High-Performance Thin Films
- Why is HR-TEM used after high-temperature heat treatment? Visualize structural evolution and material integrity.
- Why is Copper (Cu) introduced as a flux in AlN single crystal growth? Enhance Source Stability and Yield
- What role does an industrial electric furnace play in PAI? Master Thermal Preparation for Metal Matrix Composites
- Why is precise temperature control programming indispensable for SFC research? Optimize Sintering Process Success
- How does the combination of a nitrogen atmosphere and magnetic stirring benefit the dissolution stage? | KINTEK



















