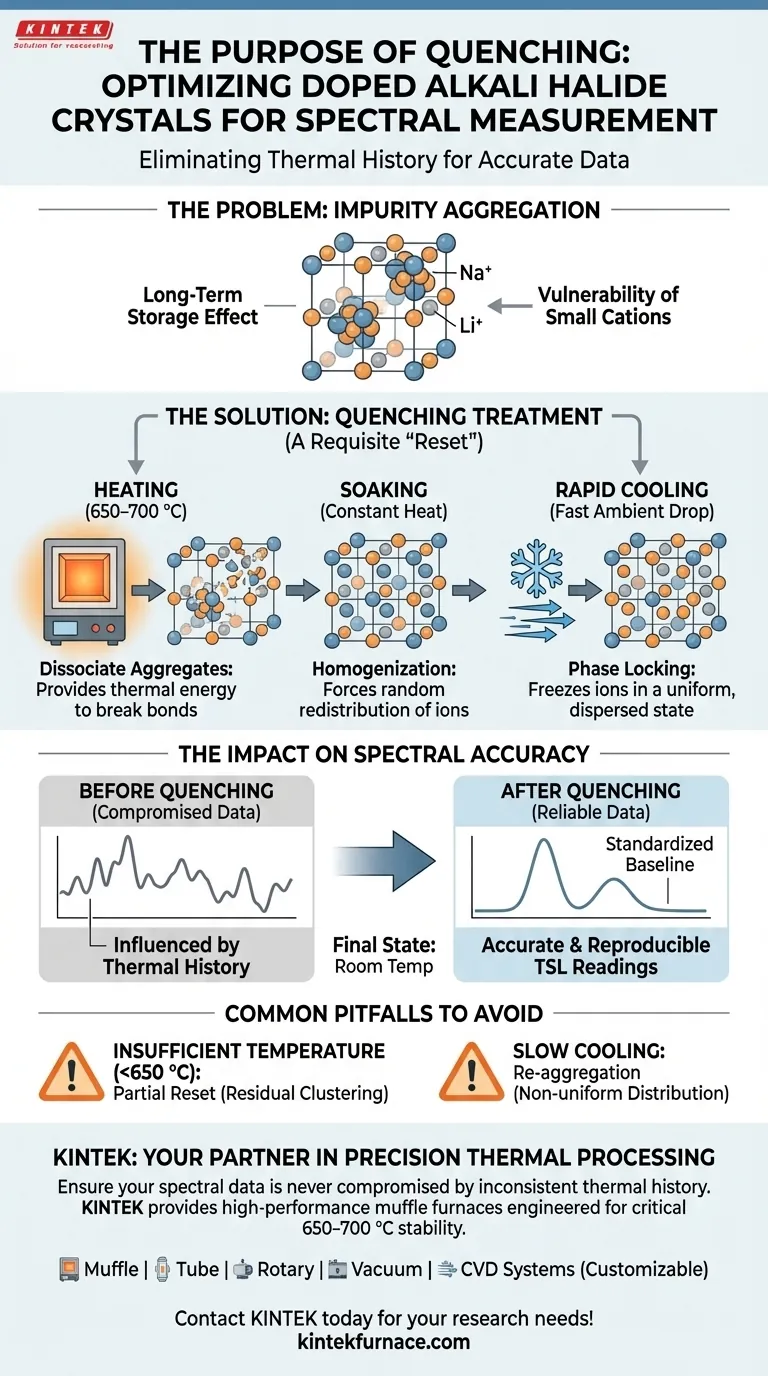
The primary purpose of quenching doped alkali halide crystals is to eliminate the physical "memory" of long-term storage and homogenize the crystal lattice. This process involves heating the samples to a critical temperature range of 650–700 °C in an electric muffle furnace, followed by rapid cooling to freeze the internal structure in a uniform state.
Quenching is a requisite "reset" mechanism that breaks up impurity aggregates, ensuring that subsequent thermoluminescence (TSL) readings reflect the material's intrinsic properties rather than its thermal history.

The Problem: Impurity Aggregation
To understand the necessity of quenching, one must first understand what happens to a crystal at rest.
The Effect of Long-Term Storage
When doped crystals sit in storage for extended periods, the impurity ions within them do not remain static.
Over time, these ions tend to migrate and cluster together, forming aggregates.
The Vulnerability of Small Cations
This clustering phenomenon is particularly prevalent with small-radius impurity cations.
Common dopants such as Li+ (Lithium) and Na+ (Sodium) are highly susceptible to forming these non-uniform clumps within the lattice structure.
The Solution: Restoring Homogeneity
The quenching treatment in the muffle furnace reverses the aggregation process.
Re-dispersing the Ions
Heating the crystal to 650–700 °C provides enough thermal energy to break the bonds holding the impurity aggregates together.
This forces the clustered ions to separate and redistribute themselves throughout the crystal volume.
Locking in Random Distribution
The subsequent rapid cooling is just as critical as the heating phase.
By dropping the temperature quickly, the impurities are trapped in their dispersed state, resulting in a uniform and random distribution across the lattice.
The Impact on Spectral Accuracy
The ultimate goal of this physical treatment is data integrity.
Eliminating Thermal History
Without quenching, a crystal’s spectral response is heavily influenced by its "thermal history"—essentially, how it was stored and the temperatures it was exposed to over time.
Quenching erases this history, providing a standardized baseline for every sample.
Ensuring TSL Accuracy
For techniques like thermoluminescence (TSL), the arrangement of impurities directly dictates the spectral output.
By ensuring the impurities are randomly distributed, the quenching process guarantees that the resulting spectra are accurate and reproducible.
Common Pitfalls to Avoid
While quenching is a corrective measure, improper execution can lead to compromised data.
Insufficient Temperature
If the furnace does not reach the critical 650–700 °C threshold, the energy supplied may be insufficient to fully dissociate the aggregates.
This results in a "partial reset," where the spectral data remains contaminated by residual clustering.
Slow Cooling Rates
If the cooling process is too gradual, the ions may have time to re-aggregate before the lattice stabilizes.
Rapid cooling is non-negotiable to maintain the random distribution achieved during heating.
Making the Right Choice for Your Project
When preparing alkali halide crystals for analysis, apply the quenching protocol based on your specific accuracy requirements.
- If your primary focus is TSL reproducibility: Ensure every sample undergoes the exact same 650–700 °C cycle to standardize the impurity distribution.
- If your primary focus is investigating storage effects: You may choose to skip quenching on a control group to deliberately measure the impact of aggregation on the spectra.
Consistency in the quenching process is the single most important factor for obtaining reliable spectral data.
Summary Table:
| Process Phase | Temperature Range | Primary Objective | Impact on Crystal Lattice |
|---|---|---|---|
| Heating | 650–700 °C | Dissociate Aggregates | Breaks bonds of clustered impurity ions |
| Soaking | Constant Heat | Homogenization | Forces random redistribution of ions |
| Rapid Cooling | Fast Ambient Drop | Phase Locking | Freezes ions in a uniform, dispersed state |
| Final State | Room Temp | Baseline Reset | Eliminates thermal history for accurate TSL |
Precision in thermal processing is the key to scientific accuracy. KINTEK provides high-performance muffle furnaces specifically engineered to reach and stabilize the critical 650–700 °C range required for crystal homogenization. Backed by expert R&D and manufacturing, we offer a comprehensive suite of Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your laboratory’s unique research needs. Ensure your spectral data is never compromised by inconsistent thermal history. Contact KINTEK today to find the perfect furnace for your research!
Visual Guide
References
- K. Shunkeyev, Zarina Serikkaliyeva. The Nature of High-Temperature Peaks of Thermally Stimulated Luminescence in NaCl:Li and KCl:Na Crystals. DOI: 10.3390/cryst15010067
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why is staged temperature control required in industrial air drying ovens for carbon nanofibers? Key Safety Insights
- How does the Flash Heating (FH) process impact the growth of REBCO films? Master Rapid Thermal Ramp Requirements
- What are the primary advantages of industrial microwave heating equipment? Enhanced Uranium Recovery Through Innovation
- Why is a constant temperature drying oven set to 60°C for 24 hours? Optimizing Sr4Al6O12SO4 Powder Quality
- What are the functions of an industrial drying furnace vertically installed below a shredder? Efficient LIB Recycling
- How do laboratory thermostatic baths contribute to the phosphoric acid treatment of 3Y-TZP ceramics? Boost Bioactivity
- What role does a nitrogen protection device play in copper-based halide thin films? Optimize Your Lab Annealing Process
- Why must temperature loss be monitored during the aluminum alloy refining cycle? Essential Tips for Casting Success



















