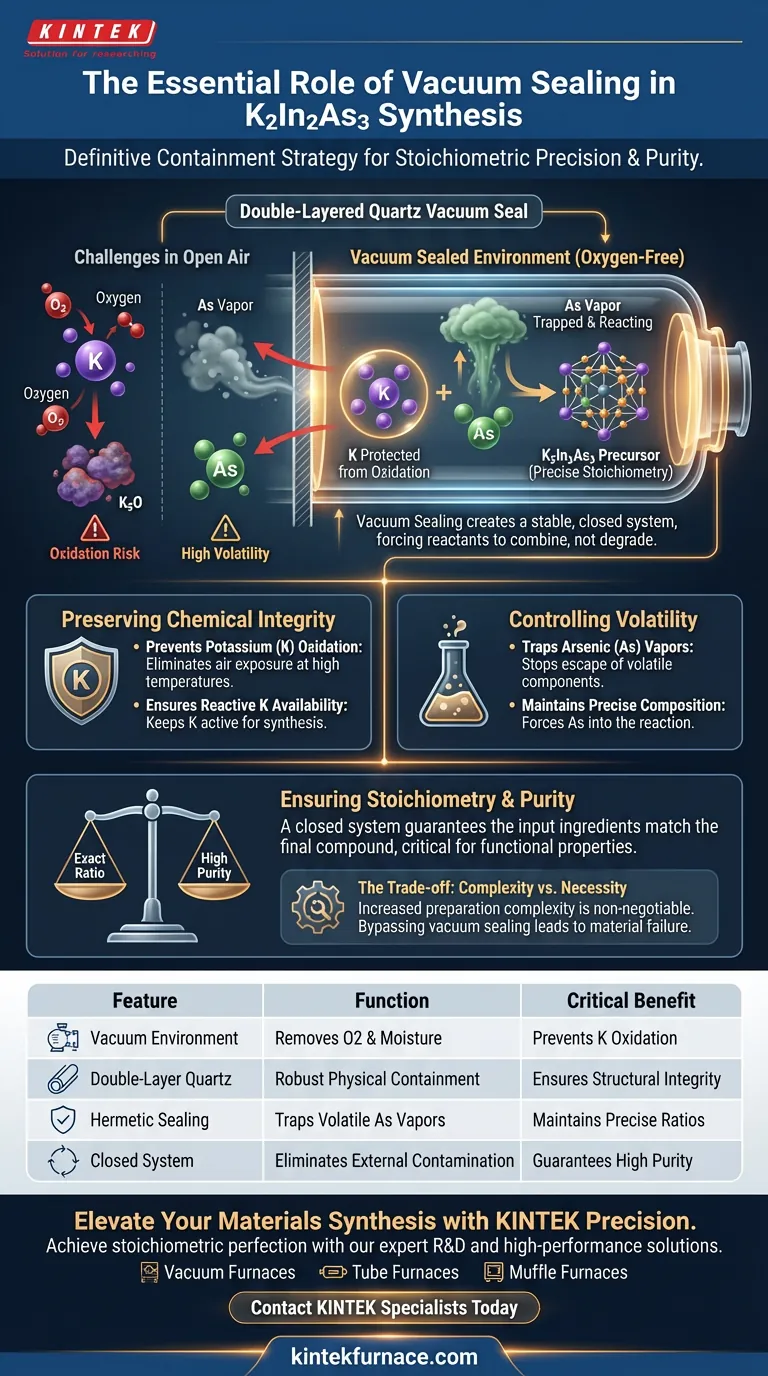
Vacuum sealing is the definitive containment strategy required to synthesize K2In2As3 precursors successfully. By utilizing double-layered quartz tubes, this technique isolates the reaction from the atmosphere, effectively preventing the oxidation of reactive potassium and trapping volatile arsenic to ensure a precise chemical composition.
The synthesis of K2In2As3 balances high reactivity with high volatility. Vacuum sealing provides the essential stable environment that forces reactants to combine rather than degrade, securing the material's stoichiometric precision and purity.

Preserving Chemical Integrity
The Reactivity of Potassium
Potassium (K) is an active alkali metal with a high affinity for oxygen. At the high temperatures required for solid-state reactions, potassium reacts aggressively if exposed to air. Vacuum sealing creates an oxygen-free environment, protecting the potassium from oxidation so it remains available for the reaction.
Controlling Arsenic Volatility
Arsenic (As) components are highly prone to volatilization, meaning they turn to vapor easily when heated. In an open or poorly sealed system, arsenic vapor would escape the reaction zone entirely. The vacuum seal traps these vapors within the tube, forcing the arsenic to participate in the synthesis rather than dissipating.
Ensuring Stoichiometry and Purity
Maintaining the Precise Ratio
The functional properties of K2In2As3 depend on a strict stoichiometric ratio between its elements. Any loss of Potassium (via oxidation) or Arsenic (via evaporation) alters this chemical balance permanently. The sealed environment acts as a closed system, ensuring that the ingredients you put in are exactly what remain in the final compound.
The Double-Layered Quartz Advantage
The process specifically utilizes double-layered quartz tubes to house the reaction. This configuration offers enhanced stability, reducing the risk of seal failure during high-temperature sintering. It provides a robust physical barrier that maintains the internal vacuum against external environmental factors.
Understanding the Trade-offs
Complexity vs. Necessity
Implementing a double-layered vacuum seal significantly increases the complexity of sample preparation compared to standard sintering. It requires specialized equipment and precise handling to ensure the quartz tubes are sealed without leaks. However, this added effort is non-negotiable; attempting to bypass vacuum sealing will invariably lead to material failure due to impurity and off-stoichiometry.
Making the Right Choice for Your Goal
To maximize the quality of your K2In2As3 synthesis, focus on these critical control points:
- If your primary focus is High Purity: Prioritize the evacuation process to remove all traces of oxygen, ensuring the active potassium remains unoxidized.
- If your primary focus is Stoichiometric Precision: Verify the integrity of the double-layered quartz seal to strictly inhibit the volatilization and escape of arsenic components.
Ultimately, vacuum sealing is not merely a precaution but the foundational requirement for stabilizing the complex chemistry of K2In2As3.
Summary Table:
| Feature | Function in K2In2As3 Synthesis | Critical Benefit |
|---|---|---|
| Vacuum Environment | Removes oxygen and moisture | Prevents reactive Potassium (K) oxidation |
| Double-Layered Quartz | Provides robust physical containment | Ensures structural integrity during sintering |
| Hermetic Sealing | Traps volatile Arsenic (As) vapors | Maintains precise stoichiometric ratios |
| Closed System | Eliminates external contamination | Guarantees high material purity and quality |
Elevate Your Materials Synthesis with KINTEK Precision
Achieving stoichiometric perfection in complex compounds like K2In2As3 requires rigorous thermal control and containment. At KINTEK, we understand the delicate balance of high-temperature reactions. Backed by expert R&D and manufacturing, we offer high-performance Vacuum, Tube, and Muffle furnaces—all customizable to your unique research needs.
Don't let oxidation or volatility compromise your results. Partner with KINTEK to secure the purity and precision your lab demands. Contact our specialists today to find the ideal high-temp solution for your next breakthrough!
Visual Guide
References
- Memristive InAs‐Based Semiconductors with Anisotropic Ion Transport. DOI: 10.1002/adma.202500056
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What are the process advantages of using solution impregnation for PtS/Ti3C2Tx? Superior In-Situ Growth vs. Mixing
- Why is temperature control precision critical for gas diffusion electrodes? Achieve Perfect PTFE Redistribution
- What is the role of calcination using high-temperature furnaces in the top-down synthesis of ZnO-NPs?
- How does a benchtop industrial oven improve efficiency? Boost Energy Savings and Space Use
- What is the purpose of hydrogen pre-treatment for Ni-Co doped carbon nanotubes? Unlock Superior Catalyst Activation
- What are the advantages of PVD equipment for solar absorber films? Achieve Nanometer Precision and Maximum Efficiency
- Why use automatic temperature compensation for Sb-Te melts? Ensure Data Accuracy with Precise Thermal Control
- What is the design focus of a thermal reactor in flash pyrolysis? Optimize Bio-oil Yield with Precision Engineering



















