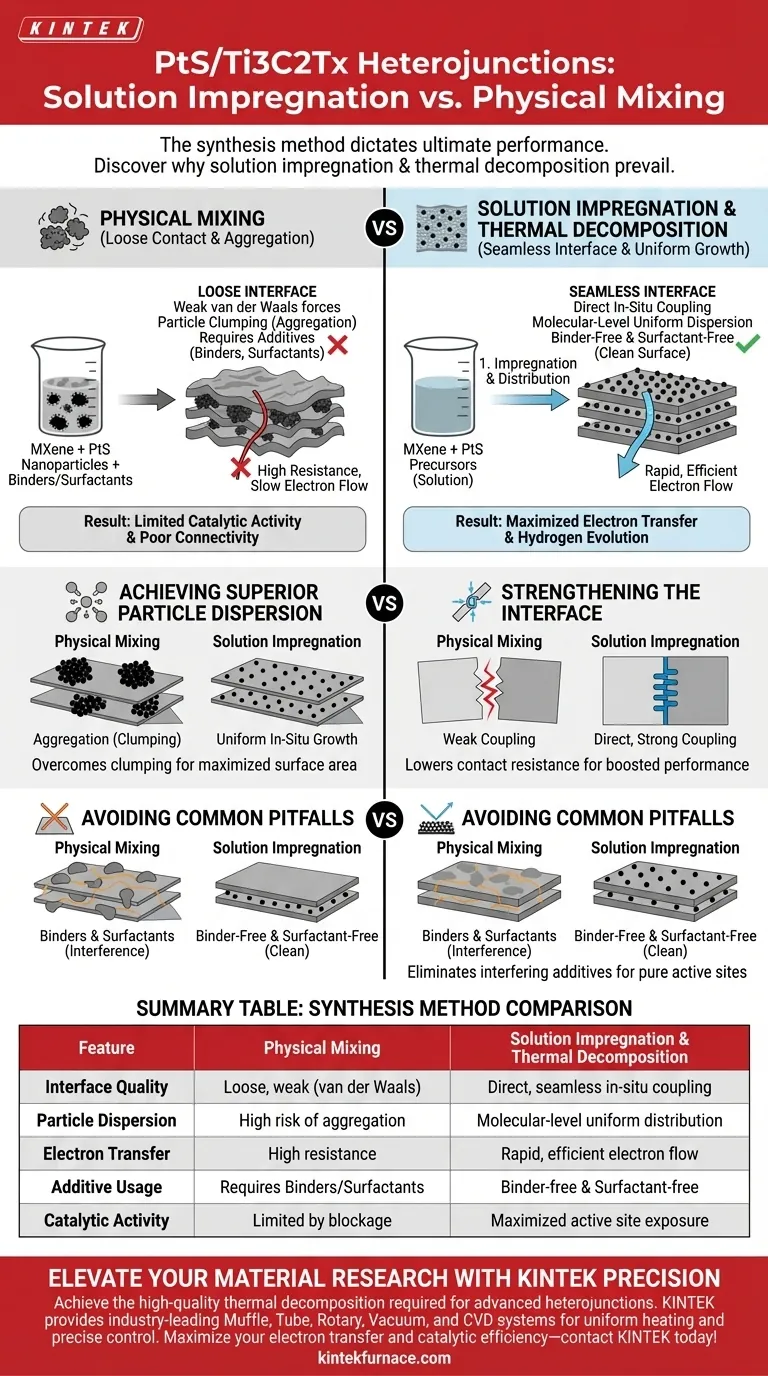
The synthesis method dictates the ultimate performance of the heterojunction. The primary advantage of using solution impregnation combined with thermal decomposition over physical mixing lies in the creation of a seamless, high-quality interface. While physical mixing often results in loose contact and aggregation, this in-situ method ensures PtS nanoparticles are grown directly onto the Ti3C2Tx MXene nanosheets, resulting in superior dispersion and electrical connectivity without the use of interfering additives.
The in-situ growth strategy creates an intimate, binder-free contact between the catalytic PtS and the conductive MXene support, which is the fundamental requirement for maximizing electron transfer and hydrogen evolution efficiency.

Achieving Superior Particle Dispersion
Overcoming Aggregation
One of the critical failures of physical mixing is the tendency for nanoparticles to clump together. By using solution impregnation, the PtS precursors are distributed evenly across the MXene surface at the molecular level before crystallization occurs.
Uniform In-Situ Growth
The subsequent thermal decomposition converts these precursors into nanoparticles right where they sit. This ensures that the final PtS nanoparticles are dispersed with high uniformity across the nanosheets, maximizing the surface area available for catalytic reactions.
Strengthening the Interface
Direct Coupling vs. Loose Contact
Physical mixing relies on weak van der Waals forces to hold components together. In contrast, the thermal decomposition process facilitates a direct growth strategy. This physical and chemical integration anchors the nanoparticles firmly to the support.
Enhancing Electron Transfer
The quality of the interface dictates how fast electrons can move. The strong interface coupling achieved through this method significantly lowers the contact resistance between the active PtS sites and the conductive MXene.
Boosting Catalytic Performance
Because electrons flow more efficiently to the active sites, the material exhibits a significant enhancement in electrocatalytic hydrogen evolution. This performance metric is difficult to replicate with the resistive interfaces common in physically mixed composites.
Avoiding Common Processing Pitfalls
Eliminating Binders
Physical mixing often requires the addition of non-conductive binders to keep the materials adhered to one another. The impregnation/decomposition method creates a robust structure without requiring additional binders, preventing the dilution of the material's conductive properties.
Removing Surfactant Interference
Surfactants are frequently used in mixing processes to stabilize particles, but they can block active catalytic sites. This direct synthesis approach creates a "clean" surface without surfactants, ensuring that every PtS nanoparticle is fully exposed and chemically active.
Making the Right Choice for Your Goal
To maximize the potential of your PtS/Ti3C2Tx heterojunctions, consider the following based on your specific engineering requirements:
- If your primary focus is maximizing catalytic activity: Use the solution impregnation method to ensure every nanoparticle is electrically connected to the support for optimal electron transfer.
- If your primary focus is surface purity: Choose this thermal decomposition route to avoid the contamination and site-blocking effects caused by binders and surfactants.
This process transforms the MXene from a simple support structure into an integrated, high-performance electron highway.
Summary Table:
| Feature | Physical Mixing | Solution Impregnation & Thermal Decomposition |
|---|---|---|
| Interface Quality | Loose, weak contact (van der Waals) | Direct, seamless in-situ coupling |
| Particle Dispersion | High risk of aggregation/clumping | Molecular-level uniform distribution |
| Electron Transfer | High resistance due to poor contact | Rapid, efficient electron flow |
| Additive Usage | Often requires binders/surfactants | Binder-free and surfactant-free |
| Catalytic Activity | Limited by surface blocking/resistance | Maximized active site exposure |
Elevate Your Material Research with KINTEK Precision
To achieve the high-quality thermal decomposition required for advanced heterojunctions like PtS/Ti3C2Tx, you need the right thermal processing equipment. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems—all backed by expert R&D and manufacturing to ensure uniform heating and precise atmosphere control.
Whether you are a laboratory researcher or an industrial manufacturer, our customizable high-temperature furnaces are designed to meet your unique synthesis needs. Maximize your electron transfer and catalytic efficiency—contact KINTEK today to find the perfect solution for your lab!
Visual Guide
References
- Young-Hee Park, Jongsun Lim. Direct Growth of Platinum Monosulfide Nanoparticles on MXene via Single‐Source Precursor for Enhanced Hydrogen Evolution Reaction. DOI: 10.1002/smsc.202500407
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
People Also Ask
- Why is a high-pressure autoclave essential for nanomaterials? Unlock Superior Crystallinity and Quantum Yield
- Why is temperature control precision critical for gear steel pseudo-carburizing? Ensure Valid Microstructural Results
- What are the uses of furnace in laboratory? The Essential Tool for Material Transformation
- What is the purpose of using a furnace at 500 °C for catalyst support pretreatment? Optimize Purity and Performance
- Why is a laboratory vacuum drying oven essential for the swelling-encapsulation-shrinkage method? Lock-in Film Quality
- What role does X-ray diffraction (XRD) play in evaluating ZIF thermal treatment? Master Material Transformation
- Why is an auxiliary gas supply device required for oil sludge pyrolysis? Ensure Stable Thermal Balance
- What are the advantages and disadvantages of microwave drying for iron ore briquettes? Expert Process Insights



















