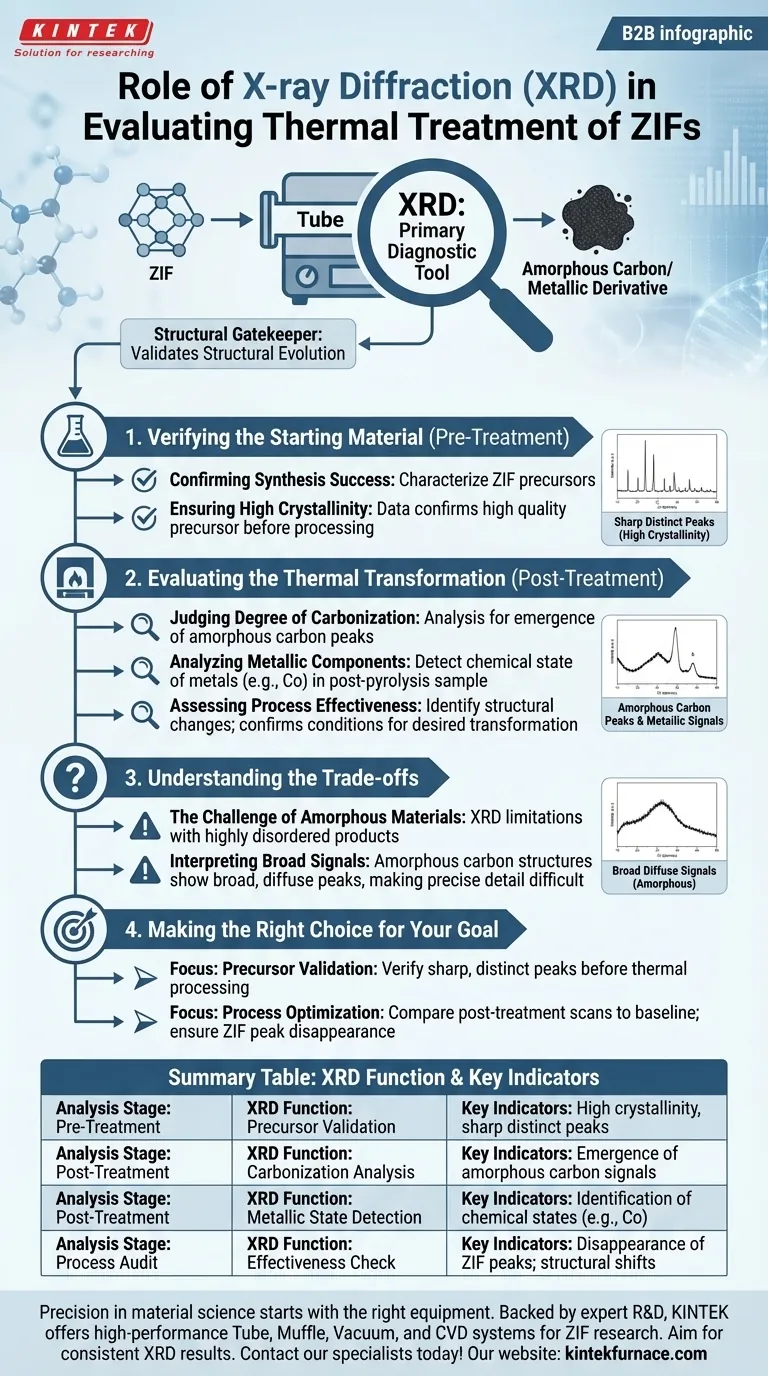
X-ray diffraction (XRD) functions as the primary diagnostic tool for validating the structural evolution of Zeolitic Imidazolate Frameworks (ZIFs) subjected to thermal treatment. It provides a comparative analysis of the material's crystallinity before and after processing in a tube furnace to determine the success of the transformation.
XRD acts as a structural gatekeeper, first confirming the quality of the ZIF precursor and subsequently verifying its conversion into amorphous carbon and metallic derivatives during pyrolysis.

Verifying the Starting Material
Confirming Synthesis Success
Before any thermal treatment begins, XRD is used to characterize the ZIF precursors.
Ensuring High Crystallinity
The data must confirm that the starting material possesses high crystallinity. This step ensures that the tube furnace process is performed on a high-quality, successfully synthesized framework rather than a defective product.
Evaluating the Thermal Transformation
Judging the Degree of Carbonization
Once the material has been processed in the tube furnace, XRD is used to analyze the resulting derivatives. Specifically, it looks for the emergence of amorphous carbon peaks, which act as a signature that the organic framework has been successfully converted.
Analyzing Metallic Components
Thermal treatment often alters the metallic nodes within the ZIF structure. XRD is capable of detecting the chemical state of these metallic components, such as Cobalt, in the post-pyrolysis sample.
Assessing Process Effectiveness
By identifying these specific structural changes, researchers can judge the effectiveness of the heat treatment. The presence of specific post-treatment peaks confirms whether the tube furnace reached the necessary conditions to drive the desired chemical and structural changes.
Understanding the Trade-offs
The Challenge of Amorphous Materials
While XRD is the standard for analyzing crystal structures, it has limitations when the product becomes highly disordered.
Interpreting Broad Signals
If the tube furnace treatment results in a fully amorphous carbon structure with no graphitic ordering or metallic crystallites, the XRD peaks may become broad and diffuse. This can make it challenging to derive precise structural details compared to the sharp peaks of the original crystalline ZIF.
Making the Right Choice for Your Goal
To effectively utilize XRD in your thermal processing workflow, match your analysis to your specific stage of development:
- If your primary focus is precursor validation: verify that your pre-treatment scan shows sharp, distinct peaks to confirm high crystallinity before wasting resources on thermal processing.
- If your primary focus is process optimization: compare post-treatment scans against the baseline to ensure the complete disappearance of ZIF peaks and the appearance of specific metal or carbon signals.
By systematically comparing these diffraction patterns, you convert raw furnace data into definitive proof of material transformation.
Summary Table:
| Analysis Stage | XRD Function | Key Indicators |
|---|---|---|
| Pre-Treatment | Precursor Validation | High crystallinity, sharp distinct peaks |
| Post-Treatment | Carbonization Analysis | Emergence of amorphous carbon signals |
| Post-Treatment | Metallic State Detection | Identification of chemical states (e.g., Co) |
| Process Audit | Effectiveness Check | Disappearance of ZIF peaks; structural shifts |
Precision in material science starts with the right equipment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Vacuum, and CVD systems—all customizable for your unique ZIF research needs. Whether you are aiming for perfect carbonization or complex metallic derivatives, our lab high-temp furnaces provide the thermal stability required for consistent XRD results. Contact our specialists today to optimize your thermal treatment process!
Visual Guide
References
- Yan Yang, Gai Zhang. Enhanced Electrocatalytic Activity for ORR Based on Synergistic Effect of Hierarchical Porosity and Co-Nx Sites in ZIF-Derived Heteroatom-Doped Carbon Materials. DOI: 10.3390/c11030070
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is the purpose of using an industrial oven for the pretreatment of reinforcement powders? | Enhance Composite Bond
- Why is a stainless steel autoclave with a Teflon liner necessary for BiVO4? Ensure Purity & High Performance
- What is the function of a high-temperature heating reactor in OPF delignification? Unlock High-Purity Cellulose
- What are the functions of a programmed temperature rise experimental system? Master Coal Pre-Oxidation Research
- Why is a furnace with programmed temperature control required for catalyst regeneration? Ensure Catalyst Stability
- What are the advantages of using a vacuum drying oven for precursors on carbon paper? Maximize Material Performance
- How should materials with high moisture content be handled before heating? Ensure Safety and Quality in Thermal Processing
- What protective roles does argon gas play in SiC sintering? Essential Insights for High-Purity Ceramics



















