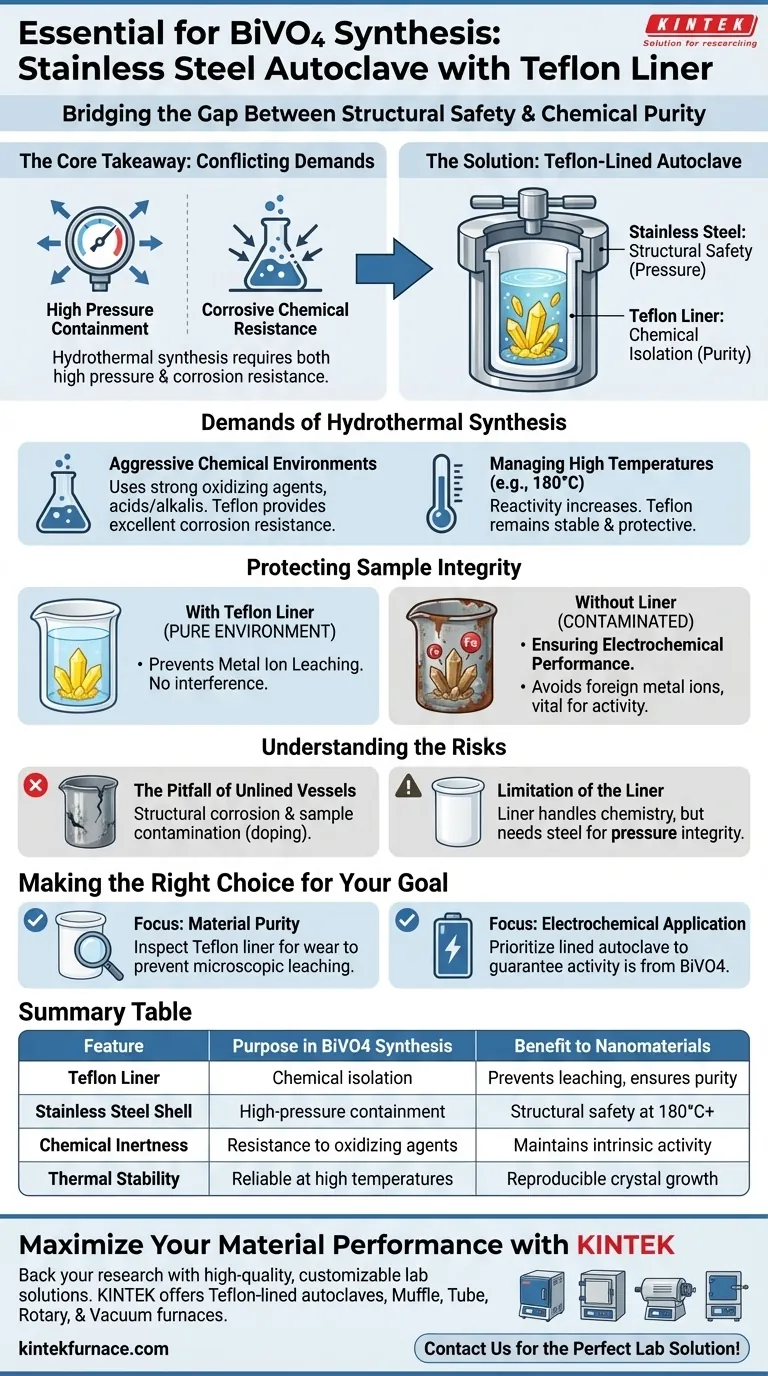
A stainless steel autoclave equipped with a Teflon liner is essential for Bismuth Vanadate (BiVO4) synthesis to bridge the gap between structural safety and chemical purity. This specific setup is required to withstand the harsh hydrothermal conditions—specifically temperatures around 180 °C combined with strong oxidizing, acidic, or alkaline agents—that would otherwise corrode bare metal and contaminate your nanomaterials.
Core Takeaway Hydrothermal synthesis places conflicting demands on equipment: the need to contain high pressure and the need to resist corrosive chemicals. The Teflon-lined autoclave solves this by using stainless steel for pressure containment and a Teflon insert for chemical isolation, ensuring the final BiVO4 crystals retain the high purity necessary for optimal electrochemical activity.

The Demands of Hydrothermal Synthesis
Withstanding Aggressive Chemical Environments
Synthesizing BiVO4 requires a reactive environment that is often hostile to standard laboratory materials. The process typically utilizes strong oxidizing agents, acids, or alkalis to drive the reaction.
Teflon provides excellent corrosion resistance and chemical inertness against these reagents. It acts as a barrier that prevents the reaction mixture from interacting with the vessel walls.
Managing High Temperatures
The synthesis process generally occurs at elevated temperatures, such as 180 °C. At this thermal level, the reactivity of chemical agents increases significantly.
Materials that might be passive at room temperature can become destructive to containment vessels under these conditions. The Teflon liner is specifically chosen because it remains stable and protective even at these elevated operating temperatures.
Protecting Sample Integrity
Preventing Metal Ion Leaching
The most critical function of the Teflon liner is the preservation of sample purity. If the synthesis solution were to touch the stainless steel shell, the metal would corrode, leaching iron or other metal ions into the mixture.
This creates a "pure environment" where the Bismuth Vanadate crystals can grow without interference.
Ensuring Electrochemical Performance
For nanomaterials like BiVO4, purity is not just about composition; it is about function. The presence of foreign metal ions can disastrously impact the material's performance.
The primary reference notes that avoiding contamination is vital for maintaining electrochemical activity. The liner ensures that the intrinsic properties of the nanomaterials are not altered by impurities derived from the reactor itself.
Understanding the Risks of Improper Equipment
The Pitfall of Unlined Vessels
Using a standard stainless steel vessel without a liner is a common error that leads to dual failure. First, the vessel itself suffers structural damage due to corrosion.
Second, and more importantly for the researcher, the experiment is compromised. The resulting BiVO4 will likely exhibit poor performance characteristics due to doping from the vessel's metal ions.
Limitation of the Liner
While the Teflon liner is crucial for chemical resistance, it relies entirely on the stainless steel shell for structural integrity.
The liner handles the chemistry, but the steel handles the physics (pressure). Both components must be in good condition to ensure the synthesis is both safe and successful.
Making the Right Choice for Your Goal
To maximize the quality of your Bismuth Vanadate nanostructures, apply these principles to your experimental design:
- If your primary focus is Material Purity: Ensure your Teflon liner is inspected for scratches or wear before every run to prevent microscopic leaching of the outer steel shell.
- If your primary focus is Electrochemical Application: prioritize the use of a lined autoclave to guarantee that any observed activity is due to the BiVO4 itself, not catalytic effects from metal contaminants.
By isolating your chemistry from your containment hardware, you ensure that your results are reproducible and your materials perform as designed.
Summary Table:
| Feature | Purpose in BiVO4 Synthesis | Benefit to Nanomaterials |
|---|---|---|
| Teflon Liner | Chemical isolation from acids/alkalis | Prevents metal ion leaching & ensures purity |
| Stainless Steel Shell | High-pressure containment | Provides structural safety at 180°C+ temperatures |
| Chemical Inertness | Resistance to strong oxidizing agents | Maintains intrinsic electrochemical activity |
| Thermal Stability | Reliable operation at high temperatures | Guarantees reproducible crystal growth conditions |
Maximize Your Material Performance with KINTEK
Don't let metal contamination compromise your hydrothermal synthesis. Backed by expert R&D and manufacturing, KINTEK offers high-quality Teflon-lined autoclaves, Muffle, Tube, Rotary, and Vacuum furnaces—all customizable for your unique lab requirements. Whether you are synthesizing BiVO4 or advanced CVD nanostructures, our systems provide the precise control and chemical isolation you need for superior results.
Ready to elevate your research? Contact us today to find the perfect lab solution!
Visual Guide
References
- Nokuthula Mekgoe, Kriveshini Pillay. Synergistic electrochemical detection of ciprofloxacin using bismuth vanadate nanocomposite-modified activated carbon derived from banana peel biomass. DOI: 10.1039/d5ma00168d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- How does a constant temperature and humidity curing chamber contribute to GCCM hydration? Optimize Material Strength
- Why is a laboratory vacuum drying oven essential for the swelling-encapsulation-shrinkage method? Lock-in Film Quality
- What mechanisms generate heat in induction heating? Discover the Science of Efficient Material Processing
- Why is a precision temperature control system essential for wood carbonization? Achieve Perfect Shape Fidelity
- What is the function of a laboratory hot air drying oven in TiO2 treatment? Ensure Uniform Nanoparticle Quality
- How does a precision pressure-controlled oxidation device increase carbon chain yield? Optimize Your Annealing Process
- Why is high-performance high-temperature heat treatment equipment essential for the 900°C calcination of copper oxide?
- Why is industrial-grade nitrogen flow introduced during the biochar pyrolysis process? Ensure Safety and Quality



















