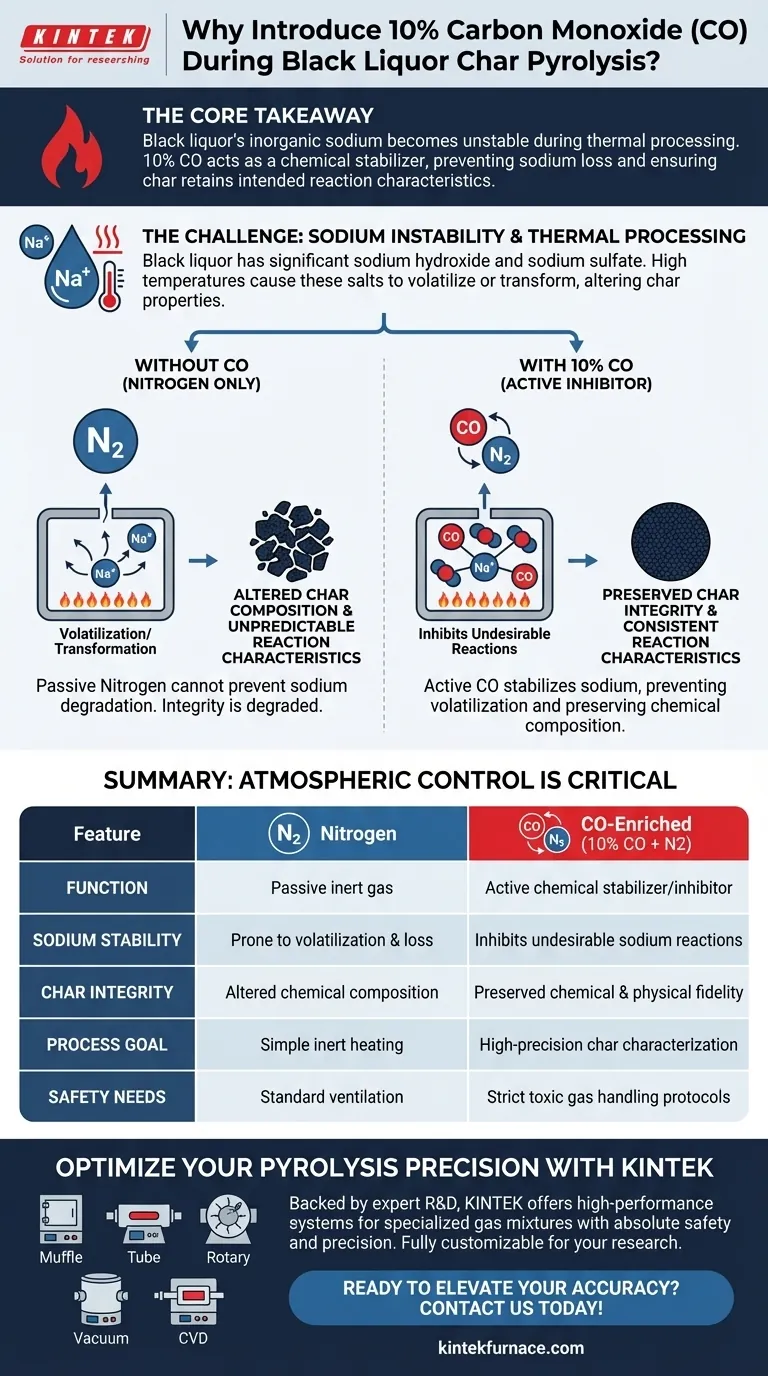
Introducing 10 percent Carbon Monoxide (CO) into a Nitrogen (N2) atmosphere during pyrolysis is a critical control measure designed to inhibit undesirable chemical reactions involving sodium. Without this specific atmospheric modification, the high temperatures inherent to pyrolysis would cause the inorganic sodium salts found in black liquor to volatilize or transform, fundamentally altering the physical and chemical properties of the resulting char.
The Core Takeaway Black liquor contains high levels of inorganic sodium which becomes unstable during thermal processing. The addition of Carbon Monoxide is not merely a buffer; it acts as a chemical stabilizer that prevents the loss or alteration of these salts, ensuring the char retains its intended reaction characteristics.

The Chemistry of Black Liquor Pyrolysis
To understand the necessity of CO, one must first understand the unique composition of the feedstock.
The Sodium Factor
Black liquor is distinct from other biomass fuels because of its significant inorganic content. It contains substantial amounts of sodium hydroxide and sodium sulfate.
Thermal Instability
These sodium salts are highly sensitive to the extreme heat required for pyrolysis. In a standard inert atmosphere, they are prone to undergoing rapid chemical changes.
The Risk of Transformation
Without inhibition, these salts may volatilize (turn into gas and escape) or transform into different compounds. This unwanted activity degrades the integrity of the sample.
Why Nitrogen Alone Is Insufficient
While Nitrogen is commonly used to displace oxygen in pyrolysis, it is passive. It cannot actively prevent the specific degradation of sodium salts.
The Role of CO as an Inhibitor
Carbon Monoxide plays an active role in the reaction chamber. By introducing a concentration of 10 percent CO, you create an environment that inhibits undesirable reactions.
Stabilizing the Components
The presence of CO effectively "locks" the sodium compounds in place. It counteracts the thermodynamic tendency of sodium to volatilize at high temperatures.
Impact on Char Quality
The ultimate goal of using this specific atmosphere is to preserve the reaction characteristics of the char for future analysis or use.
Preserving Chemical Composition
By preventing volatilization, the CO ensures that the sodium remains within the solid char matrix rather than escaping into the off-gas.
Consistent Reaction Characteristics
If the sodium chemistry changes during pyrolysis, the char will not behave predictably in subsequent processes. The CO atmosphere ensures the final product accurately reflects the material's true potential.
Operational Trade-offs
While necessary for chemical stability, introducing Carbon Monoxide requires careful consideration.
Accuracy vs. Simplicity
Using a pure Nitrogen atmosphere is simpler and safer due to the inert nature of the gas. However, this simplicity comes at the cost of chemical accuracy regarding sodium retention.
Handling Requirements
Carbon Monoxide is a toxic gas. Its use necessitates stricter safety protocols and gas handling infrastructure compared to using Nitrogen alone.
Making the Right Choice for Your Goal
The decision to use a CO-enriched atmosphere depends on the precision required for your end product.
- If your primary focus is chemical fidelity: You must use the 10 percent CO mixture to prevent the volatilization of sodium salts and preserve the char’s inorganic composition.
- If your primary focus is analyzing reaction kinetics: The CO atmosphere is essential to ensure the char's reaction characteristics are not artificially altered during the heating phase.
For precise black liquor char characterization, atmospheric control is as critical as temperature control.
Summary Table:
| Feature | Nitrogen (N2) Atmosphere | CO-Enriched Atmosphere (10% CO + N2) |
|---|---|---|
| Function | Passive inert gas | Active chemical stabilizer/inhibitor |
| Sodium Stability | Prone to volatilization and loss | Inhibits undesirable sodium reactions |
| Char Integrity | Altered chemical composition | Preserved chemical and physical fidelity |
| Process Goal | Simple inert heating | High-precision char characterization |
| Safety Needs | Standard ventilation | Strict toxic gas handling protocols |
Optimize Your Pyrolysis Precision with KINTEK
Maintaining strict atmospheric control is the only way to ensure chemical fidelity in complex processes like black liquor char production. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to handle specialized gas mixtures with absolute safety and precision. Whether you are researching biomass fuels or advanced material transformations, our lab high-temp furnaces are fully customizable to meet your unique research needs.
Ready to elevate your thermal processing accuracy? Contact us today to discuss your custom furnace requirements.
Visual Guide
References
- F. Bueno, José Luis Sánchez. CO₂ Gasification of Black Liquor Char under isothermal and dynamic conditions. DOI: 10.26754/jji-i3a.202512008
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
People Also Ask
- Why is thermal insulation applied to cylindrical components in thermal stress tests? Enhance Calculation Precision
- What is the purpose of the rapid quenching process? Capture Precise High-Pressure Data Instantly
- Why is determining the hypercooling limit necessary when measuring the heat of fusion? Optimize Your Material Research
- What types of labs benefit most from benchtop industrial ovens? Maximize Space and Efficiency in Your Lab
- What are the technical advantages of using high-purity hydrogen as a protective atmosphere? Boost Heat Treatment Speed
- What is the necessity of the subsequent pyrolysis step in ZnS-CFC preparation? Unlocking High-Performance Carbonization
- Why is Magnesium Hydride (MgH2) preferred for SiOx pre-magnesiation? Optimize Thermal Control and Battery Stability
- How does environmental control equipment assist in assessing CMS membranes? Unlock Precision in Physical Aging Tests



















