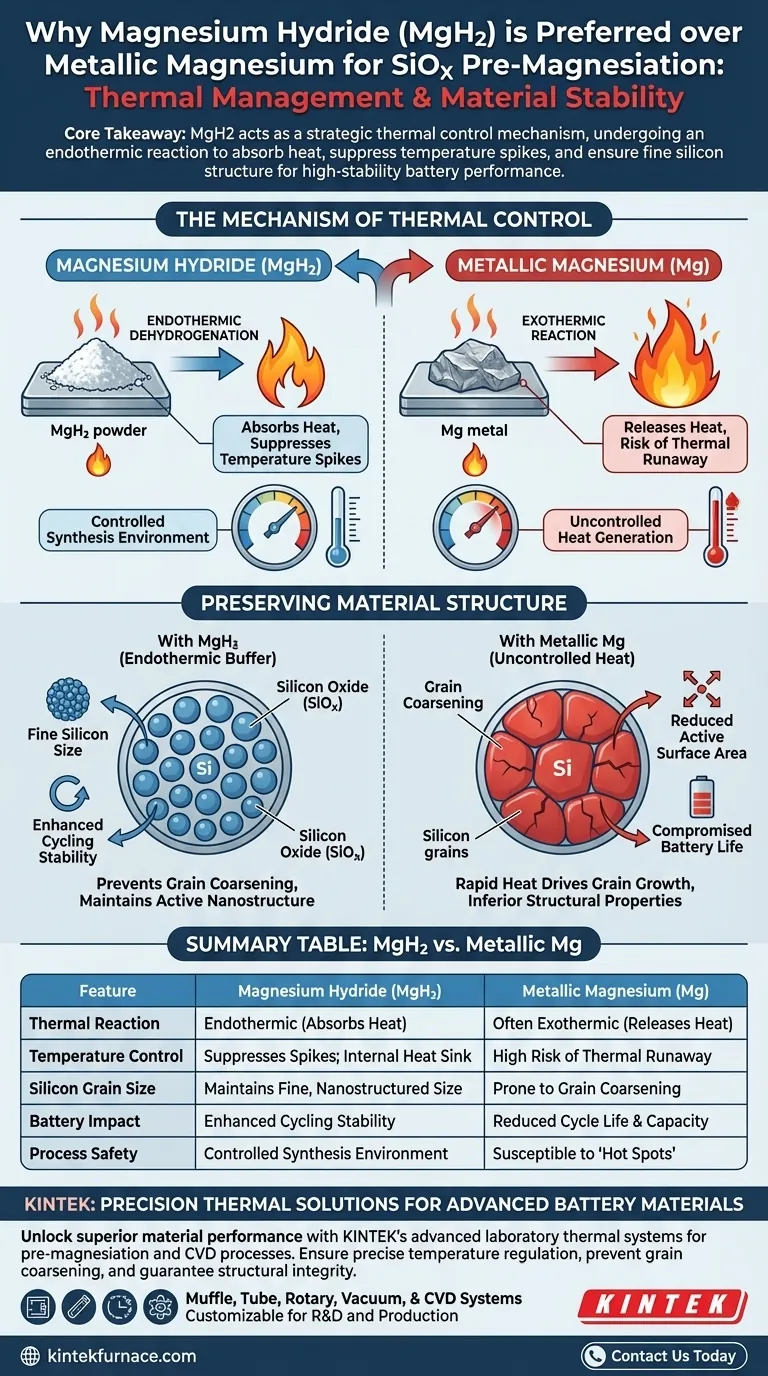
Magnesium Hydride (MgH2) is preferred primarily for its unique thermal management capabilities. Unlike metallic magnesium, the decomposition (dehydrogenation) of MgH2 is an endothermic process. This allows the material to act as an internal heat sink during heat treatment, absorbing excess energy and effectively neutralizing the risks associated with high-temperature synthesis.
Core Takeaway The selection of Magnesium Hydride acts as a strategic thermal control mechanism. By undergoing an endothermic reaction, MgH2 absorbs heat to suppress temperature spikes, preventing silicon grain coarsening and ensuring the fine structural integrity required for high-stability battery performance.

The Mechanism of Thermal Control
The Role of Endothermic Dehydrogenation
The fundamental advantage of MgH2 is its reaction to heat. As the material undergoes heat treatment, it decomposes to release hydrogen.
Crucially, this decomposition is endothermic, meaning it consumes heat from the surrounding environment. This stands in sharp contrast to exothermic reactions, which release heat and can lead to thermal runaway or "hot spots" within the material mixture.
Suppressing Temperature Spikes
During the pre-magnesiation process, maintaining a stable temperature profile is essential. The heat absorption provided by MgH2 effectively suppresses intense temperature spikes.
By moderating the internal temperature of the reaction, MgH2 ensures a controlled synthesis environment. This prevents the reaction kinetics from accelerating uncontrollably, which is a common risk when using reactants that do not offer this thermal buffering effect.
Preserving Material Structure
Preventing Grain Coarsening
Temperature control is not just a safety feature; it dictates the physical structure of the final material. High temperatures typically cause grains to merge and grow larger, a process known as grain coarsening.
If the silicon phase coarsens, the active surface area decreases, and the material's ability to accommodate volume changes during battery cycling is compromised. MgH2 prevents this by keeping temperatures in check.
Ensuring Fine Silicon Size
The goal of using MgH2 is to maintain a fine size of active silicon.
By preventing thermal spikes that lead to growth, the silicon remains in a highly active, nanostructured state. This fine structure is directly responsible for enhancing the cycling stability of the resulting SiOx anode material, leading to a longer-lasting battery.
The Risks of Alternative Sources
Uncontrolled Exothermic Reactions
While the primary reference highlights the benefits of MgH2, it implicitly outlines the pitfalls of using alternatives like metallic magnesium without a buffering mechanism.
Without the endothermic buffer of MgH2, the reaction environment is susceptible to rapid heat generation. This uncontrolled heat drives the very grain coarsening that engineers aim to avoid, resulting in a battery material with inferior structural properties and reduced cycle life.
Making the Right Choice for Your Goal
When designing synthesis protocols for Silicon Oxide anodes, the choice of precursor dictates the quality of the final architecture.
- If your primary focus is Cycling Stability: Prioritize MgH2 to maintain the fine silicon grain size necessary for long-term endurance.
- If your primary focus is Process Control: Utilize MgH2 to act as an internal thermal buffer, mitigating the risk of temperature spikes during heat treatment.
Control the temperature at the microscopic level, and you control the performance of the final cell.
Summary Table:
| Feature | Magnesium Hydride (MgH2) | Metallic Magnesium (Mg) |
|---|---|---|
| Thermal Reaction | Endothermic (Absorbs Heat) | Often Exothermic (Releases Heat) |
| Temperature Control | Suppresses spikes; internal heat sink | High risk of thermal runaway |
| Silicon Grain Size | Maintains fine, nanostructured size | Prone to grain coarsening |
| Battery Impact | Enhanced cycling stability | Reduced cycle life & capacity |
| Process Safety | Controlled synthesis environment | Susceptible to "hot spots" |
Precision Thermal Solutions for Advanced Battery Materials
Unlock superior material performance with KINTEK’s advanced laboratory thermal systems. Whether you are conducting pre-magnesiation of SiOx or complex CVD processes, our equipment ensures the precise temperature regulation required to prevent grain coarsening and ensure structural integrity.
Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your specific research or production needs.
Ready to stabilize your synthesis process? Contact us today to consult with our experts and find the perfect high-temperature solution for your lab.
Visual Guide
References
- Hyunsik Yoon, Hansu Kim. Magnesiated Si‐Rich SiO<sub><i>x</i></sub> Materials for High‐Performance Lithium‐Ion Batteries. DOI: 10.1002/batt.202500473
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Spark Plasma Sintering SPS Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What are the benefits of using graphite or stainless steel crucibles for Rubidium Chloride? Ensure Maximum Purity
- What is the function of a vacuum drying oven in SFRP processing? Preserve Material Integrity & Prevent Degradation
- How does a vacuum pressure impregnation tank achieve deep treatment? Master Advanced Wood Modification Methods
- Why is a vacuum oven preferred for drying MXene-modified electrodes? Optimize Your Lab's Electrochemical Success
- How does the pulling and rotation control system of a Czochralski growth furnace affect crystal quality?
- What function does high-purity argon gas serve in BPEA PVT preparation? Ensure High-Quality Crystal Growth
- What is the objective of GC-MS analysis on bio-oil? Unlock Chemical Value and Industrial Utility
- What is the design focus of a thermal reactor in flash pyrolysis? Optimize Bio-oil Yield with Precision Engineering












