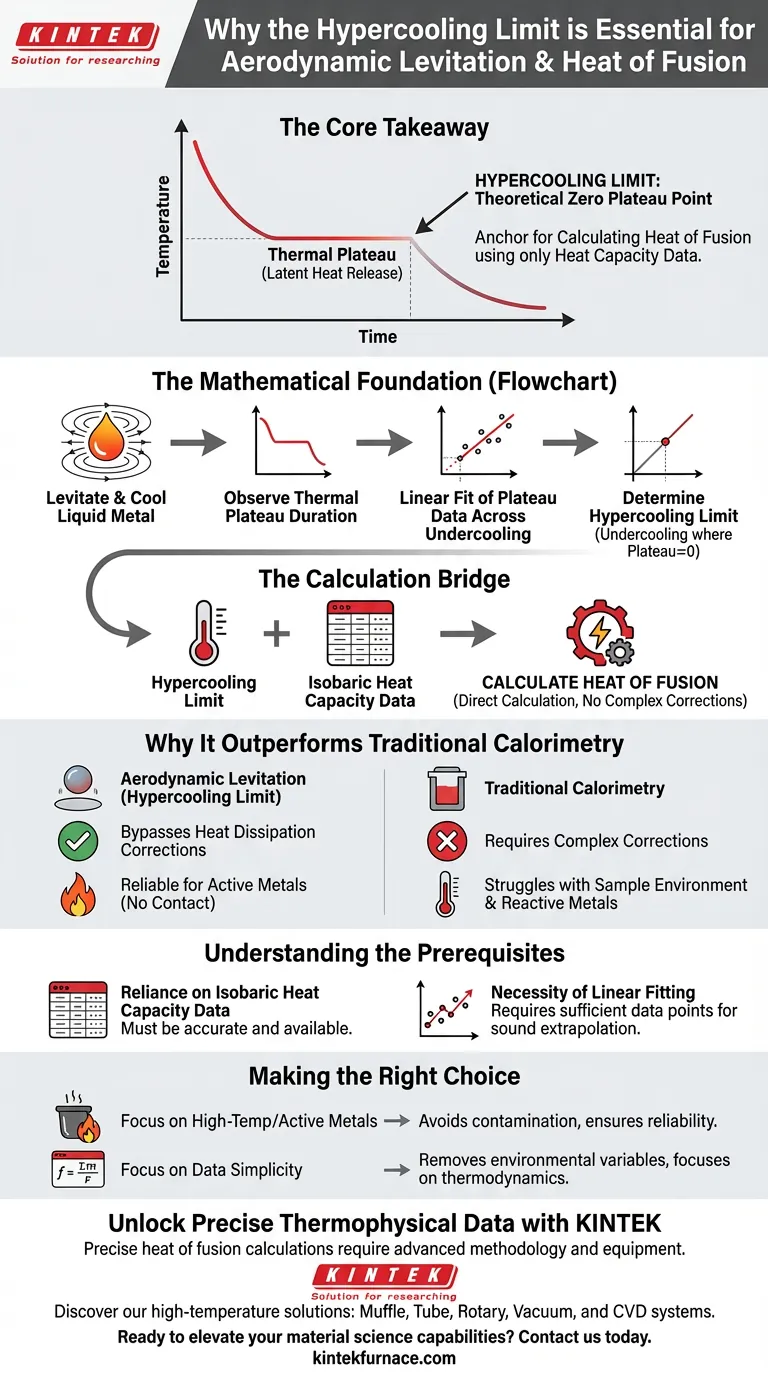
Determining the hypercooling limit is the essential mathematical anchor required to calculate the heat of fusion when using aerodynamic levitation. Without identifying this specific theoretical limit, researchers cannot convert observed cooling behaviors into precise energy values, particularly for metals that are difficult to measure using standard methods.
Core Takeaway The hypercooling limit defines the theoretical temperature at which the thermal plateau during solidification disappears. By identifying this point, researchers can calculate the heat of fusion using only heat capacity data, effectively bypassing the complex and error-prone heat dissipation corrections required by traditional calorimetry.
The Mathematical Foundation
To understand why this limit is necessary, one must look at how aerodynamic levitation uses temperature data to derive energy values.
The Role of the Thermal Plateau
When a levitated liquid metal cools and begins to solidify, it releases latent heat. This release creates a temporary stabilization in temperature known as a thermal plateau. Researchers using this method gather data on how long this plateau lasts across various levels of undercooling.
Extrapolating the Limit
The data regarding plateau duration is not used in isolation. Researchers apply a linear fit to these measurements to establish a trend. The goal is to identify the specific undercooling temperature where the thermal plateau duration theoretically drops to zero. This point is the hypercooling limit.
The Calculation Bridge
The hypercooling limit is not the final result; it is the key variable in the equation. Once determined, this limit is combined with known isobaric heat capacity data. This mathematical combination allows for the direct calculation of the metal's heat of fusion.
Why This Method Outperforms Traditional Calorimetry
The necessity of the hypercooling limit stems from the specific advantages it offers over older measurement techniques.
Bypassing Heat Dissipation Corrections
Traditional calorimetry often struggles with the environment surrounding the sample. In those methods, researchers must mathematically correct for how heat is lost to the container or surroundings. The hypercooling limit approach relies on internal thermodynamic properties, eliminating the need for these complex heat dissipation corrections.
Reliability for Active Metals
Highly active metals are chemically reactive and difficult to contain. Aerodynamic levitation isolates the material, but isolation makes direct contact measurement impossible. By relying on the mathematical foundation of the hypercooling limit, researchers can generate highly reliable thermophysical data without physically probing the volatile sample.
Understanding the Prerequisites
While this method simplifies the calculation of heat of fusion, it relies on specific data dependencies that must be managed carefully.
Reliance on Isobaric Heat Capacity
The hypercooling limit cannot be used in a vacuum. The calculation is strictly dependent on the availability of accurate isobaric heat capacity data. If the heat capacity of the specific metal is unknown or inaccurate, finding the hypercooling limit will not yield a correct heat of fusion.
The Necessity of Linear Fitting
The accuracy of the result depends on the quality of the linear fit. Researchers must collect sufficient data points across various undercooling levels to ensure the extrapolation to the "zero plateau" point (the limit) is statistically sound.
Making the Right Choice for Your Research
When deciding whether to utilize the hypercooling limit method for your project, consider your material constraints.
- If your primary focus is High-Temperature/Active Metals: This method is necessary to avoid contamination issues and container reactions while ensuring data reliability.
- If your primary focus is Data Simplicity: This approach is ideal as it removes the variable of environmental heat loss, focusing purely on the thermodynamics of the material.
By establishing the hypercooling limit, you convert a complex physical observation into a precise, mathematically derived energy value.
Summary Table:
| Feature | Aerodynamic Levitation (Hypercooling Limit) | Traditional Calorimetry |
|---|---|---|
| Core Mechanism | Mathematical extrapolation of thermal plateau | Direct measurement of energy release |
| Environment | Containerless / Non-contact | Physical container / Direct contact |
| Key Dependency | Isobaric heat capacity data | Heat dissipation corrections |
| Best For | Active, high-temperature metals | Stable, lower-temperature materials |
| Main Advantage | Eliminates environmental heat loss variables | Established, standard instrumentation |
Unlock Precise Thermophysical Data with KINTEK
Precise heat of fusion calculations require both advanced methodology and the right equipment. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of high-temperature solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are working with active metals or complex high-temperature alloys, our laboratory furnaces are fully customizable to meet your unique research needs.
Ready to elevate your material science capabilities? Contact us today to find the perfect furnace for your laboratory and experience the KINTEK advantage in precision and reliability.
Visual Guide
References
- Kanta Kawamoto, Hidekazu Kobatake. Development of Heat-of-fusion Measurement for Metals Using a Closed-type Aerodynamic Levitator. DOI: 10.2355/isijinternational.isijint-2024-053
This article is also based on technical information from Kintek Furnace Knowledge Base .
People Also Ask
- Why Use a Vacuum Oven for Cu-Cu2O/g-C3N4 Catalysts? Preserve Purity and Structural Integrity
- Why is it important to choose the right type of heat treatment furnace? Boost Efficiency and Quality in Your Lab or Facility
- What is the purpose of a microwave digestion furnace? Unlock Precise ICP-MS Results through Matrix Destruction
- Why is a furnace with high-precision temperature control required for DPKB-S? Ensuring Material Synthesis Accuracy
- What processes can continuous furnaces perform in a single step? Master Debinding and Sintering for High-Volume Production
- What are the drawbacks of cold compacting and sintering? Higher Porosity and Weaker Mechanical Properties
- How does temperature control affect nanoporous copper dealloying? Master Pore Uniformity and Size
- Why must thermal analysis equipment support multiple heating rates? Key to 5AT & NaIO4 Kinetic Studies