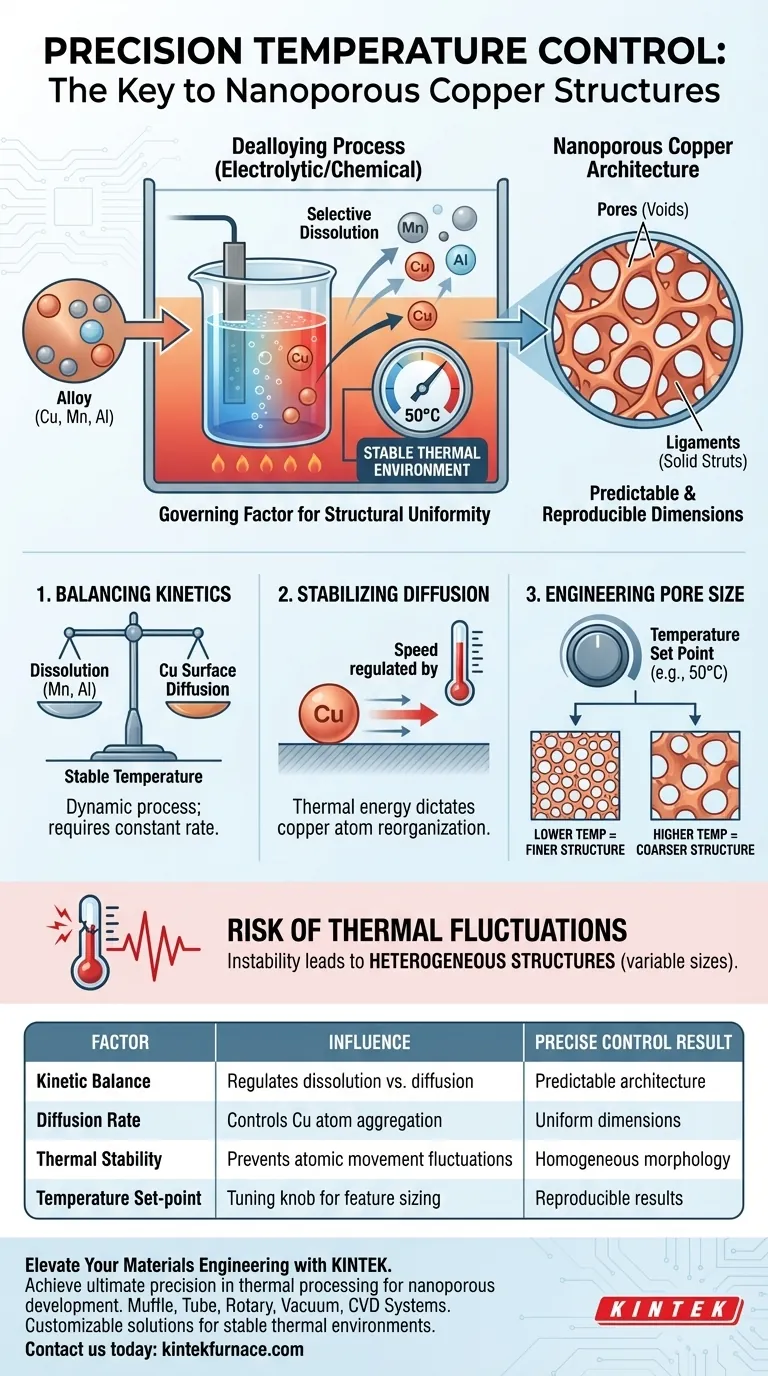
Precise temperature control is the governing factor for structural uniformity. By maintaining a stable thermal environment, you directly regulate the kinetic processes of dealloying. This stability ensures that the selective dissolution of sacrificial elements (Manganese and Aluminum) and the subsequent surface diffusion of Copper occur at a constant rate, resulting in predictable nanoporous architectures.
Dealloying is fundamentally a kinetic race between dissolution and diffusion. Controlling temperature does not just facilitate the reaction; it stabilizes the atomic movement required to engineer specific pore and ligament sizes.

The Mechanics of Thermal Stability
Balancing Kinetic Processes
Dealloying is not a static event; it is a dynamic, kinetic process. It involves the selective removal of Manganese and Aluminum from the alloy matrix. Simultaneously, the remaining Copper atoms must reorganize themselves to form the final structure.
The Role of Copper Diffusion
As the sacrificial elements dissolve, Copper atoms undergo surface diffusion. This movement is necessary to aggregate the remaining copper into a coherent network. The rate at which these atoms move is dictated almost entirely by thermal energy.
Stabilizing the Environment
Precise control creates a stable thermal environment, such as a constant 50 degrees Celsius. Without this stability, the rate of copper diffusion would fluctuate. This consistency is the only way to ensure the reaction proceeds uniformly across the entire sample.
Engineering Pore and Ligament Size
Defining the Architecture
The resulting material is defined by two features: pores (voids) and ligaments (solid struts). The dimensions of these features are not random; they are a direct result of how fast the copper atoms could move and clump together.
Dialing in Dimensions
By locking the temperature, you effectively lock the diffusion rate. This allows for the creation of structures with controllable sizes. If the temperature is held constant, the resulting pore and ligament sizes become predictable and reproducible.
Understanding the Trade-offs
The Risk of Thermal Fluctuations
The primary pitfall in dealloying is thermal instability. If the temperature spikes or drops during the process, the diffusion rate changes instantly. This leads to heterogeneous structures, where pore sizes vary significantly from one region to another.
Sensitivity to Set Points
While stability is key, the specific temperature chosen (e.g., 50 degrees Celsius) acts as a tuning knob. It is critical to note that "precise control" implies maintaining the chosen temperature, not just heating the sample. Deviating from the optimal set point can result in structures that are either too coarse or incompletely formed.
Making the Right Choice for Your Goal
To achieve high-quality nanoporous copper structures, you must view temperature as a design parameter rather than a simple environmental condition.
- If your primary focus is Structural Uniformity: Prioritize thermal insulation and feedback loops to ensure the temperature never deviates from your set point.
- If your primary focus is Feature Sizing: Experiment with different stable temperature plateaus (e.g., 50°C vs. 60°C) to alter the diffusion rate and shift the resulting pore dimensions.
Mastering the thermal environment is the first step toward mastering the material's morphology.
Summary Table:
| Factor | Influence on Nanoporous Structure | Result of Precise Control |
|---|---|---|
| Kinetic Balance | Regulates dissolution vs. surface diffusion | Predictable structural architecture |
| Diffusion Rate | Controls how Copper atoms aggregate | Uniform pore and ligament dimensions |
| Thermal Stability | Prevents fluctuations in atomic movement | Homogeneous morphology across sample |
| Temperature Set-point | Acts as a tuning knob for feature sizing | Reproducible results for specific applications |
Elevate Your Materials Engineering with KINTEK
Achieve the ultimate precision in thermal processing with KINTEK’s high-performance laboratory solutions. Backed by expert R&D and manufacturing, we provide high-temperature Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet the rigorous demands of nanoporous structure development.
Whether you are refining copper diffusion rates or scaling specialized dealloying processes, our equipment ensures the stable thermal environment your research requires. Contact us today to find the perfect furnace for your lab!
Visual Guide
References
- Jinyi Wang, Yuan Ji. Nanoporous Copper Fabricated by Dealloying Single-Phase Mn-Cu-Al Alloy and Its Non-Enzymatic Glucose Detection. DOI: 10.3390/cryst15060563
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does a gas mass flow control system (MFC) prevent copper foil adhesion? Mastering Atmospheric Purity
- What are the benefits of cold compacting and sintering? Boost Efficiency and Cut Costs in Manufacturing
- What role does fluorination process equipment play in the pretreatment of LiF-BeF2 salts? Ensure High-Purity Substrates
- How do atomizers and furnaces function in Spray Pyrolysis? Master Nanoparticle Synthesis
- What are the two key phenomena essential to understanding induction heating? Master the Core Principles
- What is the purpose of heating a precursor solution to 80 °C and 300 rpm stirring? Achieve High-Entropy Uniformity
- What are the advantages of combining vacuum hot rolling with small hole vacuuming? High-Bonding Clad Plate Production
- How do precision temperature-controlled ovens function for SiC-Ti3SiC2 preform curing? Expert Thermal Control Guide



















