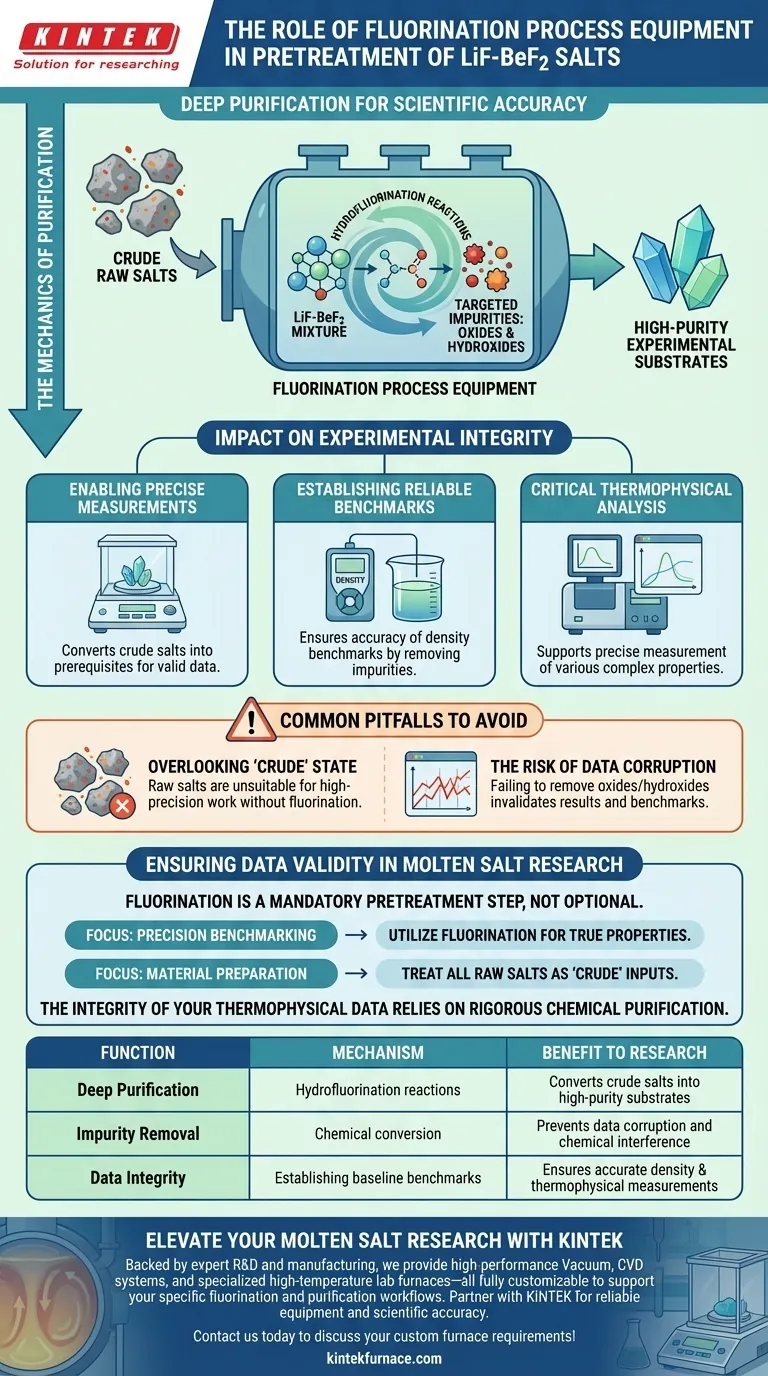
The primary role of fluorination process equipment is to execute deep purification of LiF-BeF2 salts. By leveraging hydrofluorination reactions, this equipment chemically strips away oxide and hydroxide impurities, converting crude raw materials into the high-purity substrates required for accurate scientific measurement.
The core function of this equipment is to bridge the gap between crude raw salts and experimental-grade materials. It ensures that residual impurities do not corrupt critical data, specifically safeguarding the accuracy of thermophysical property measurements and density benchmarks.

The Mechanics of Purification
Utilizing Hydrofluorination Reactions
The equipment relies on hydrofluorination to treat the salt mixture. This is not merely a physical filtration but a chemical transformation designed to deeply purify the material structure.
Targeting Specific Impurities
The process specifically targets residual oxides and hydroxides. By chemically removing these contaminants, the equipment prevents them from altering the fundamental chemistry of the LiF-BeF2 mixture.
The Impact on Experimental Integrity
Enabling Precise Measurements
Raw salts are often too crude for sensitive experimentation. Fluorination converts these salts into high-purity experimental substrates, which are absolute prerequisites for obtaining valid data.
Establishing Reliable Benchmarks
The ultimate goal of this pretreatment is to ensure the accuracy of density benchmarks. Without the removal of lighter or heavier impurities, the baseline physical properties of the salt cannot be established with confidence.
Critical Thermophysical Analysis
Beyond density, the purity achieved by this equipment supports the precise measurement of various thermophysical properties. Impurities left in the salt would otherwise introduce variables that skew these complex measurements.
Common Pitfalls to Avoid
Overlooking the "Crude" State of Raw Salts
A common error is assuming raw LiF-BeF2 salts are ready for immediate testing. You must recognize that without this specific fluorination pretreatment, the material remains in a crude state unsuitable for high-precision work.
The Risk of Data Corruption
Failing to remove oxides and hydroxides does not just lower quality; it invalidates results. Any data collected regarding density or thermal properties from unpurified salts should be considered compromised and unreliable for benchmarking.
Ensuring Data Validity in Molten Salt Research
To ensure your experimental results are credible, you must view fluorination as a mandatory pretreatment step rather than an optional enhancement.
- If your primary focus is Precision Benchmarking: You must utilize fluorination to eliminate oxides and hydroxides, ensuring your density benchmarks reflect the true properties of the salt.
- If your primary focus is Material Preparation: You should treat all raw salts as "crude" inputs that require hydrofluorination to be converted into usable experimental substrates.
The integrity of your thermophysical data relies entirely on the rigorous chemical purification provided by this equipment.
Summary Table:
| Function | Mechanism | Benefit to Research |
|---|---|---|
| Deep Purification | Hydrofluorination reactions | Converts crude salts into high-purity substrates |
| Impurity Removal | Chemical conversion of oxides/hydroxides | Prevents data corruption and chemical interference |
| Data Integrity | Establishing baseline benchmarks | Ensures accurate density and thermophysical measurements |
Elevate Your Molten Salt Research with KINTEK
Precise thermophysical data starts with uncompromising purity. At KINTEK, we understand that raw salts require rigorous pretreatment to yield valid experimental results. Backed by expert R&D and manufacturing, we provide high-performance Vacuum, CVD systems, and specialized high-temperature lab furnaces—all fully customizable to support your specific fluorination and purification workflows.
Don't let crude material impurities compromise your density benchmarks. Partner with KINTEK for the reliable equipment you need to ensure scientific accuracy. Contact us today to discuss your custom furnace requirements!
Visual Guide
References
- Jisue Moon, Theodore M. Besmann. Density Measurements of Molten LiF–BeF<sub>2</sub> and LiF–BeF<sub>2</sub>–LaF<sub>3</sub> Salt Mixtures by Neutron Radiography. DOI: 10.1021/acsomega.4c01446
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- Why is thermal growth oxidation preferred for SiO2 gate dielectrics? Superior Quality for a-IGZO TFTs
- Why is precise temperature rate control in a sintering furnace vital for ceramic-sapphire composite production?
- What role does an electric heating industrial furnace play in biomass pyrolysis? Unlock High-Quality Biochar Yields
- What performance characteristics are required for a stainless steel tubular autoclave or reactor used in SCWG?
- What role does a laboratory circulating air drying oven play in the post-treatment of composite membranes? Master Stability
- How does a constant temperature and humidity curing chamber contribute to GCCM hydration? Optimize Material Strength
- Why is precision constant temperature control required during the hardening stage of geopolymer mortar? Guide to Success
- How are impurity levels controlled during tantalum powder synthesis? Master High-Purity Magnesiothermic Reduction



















