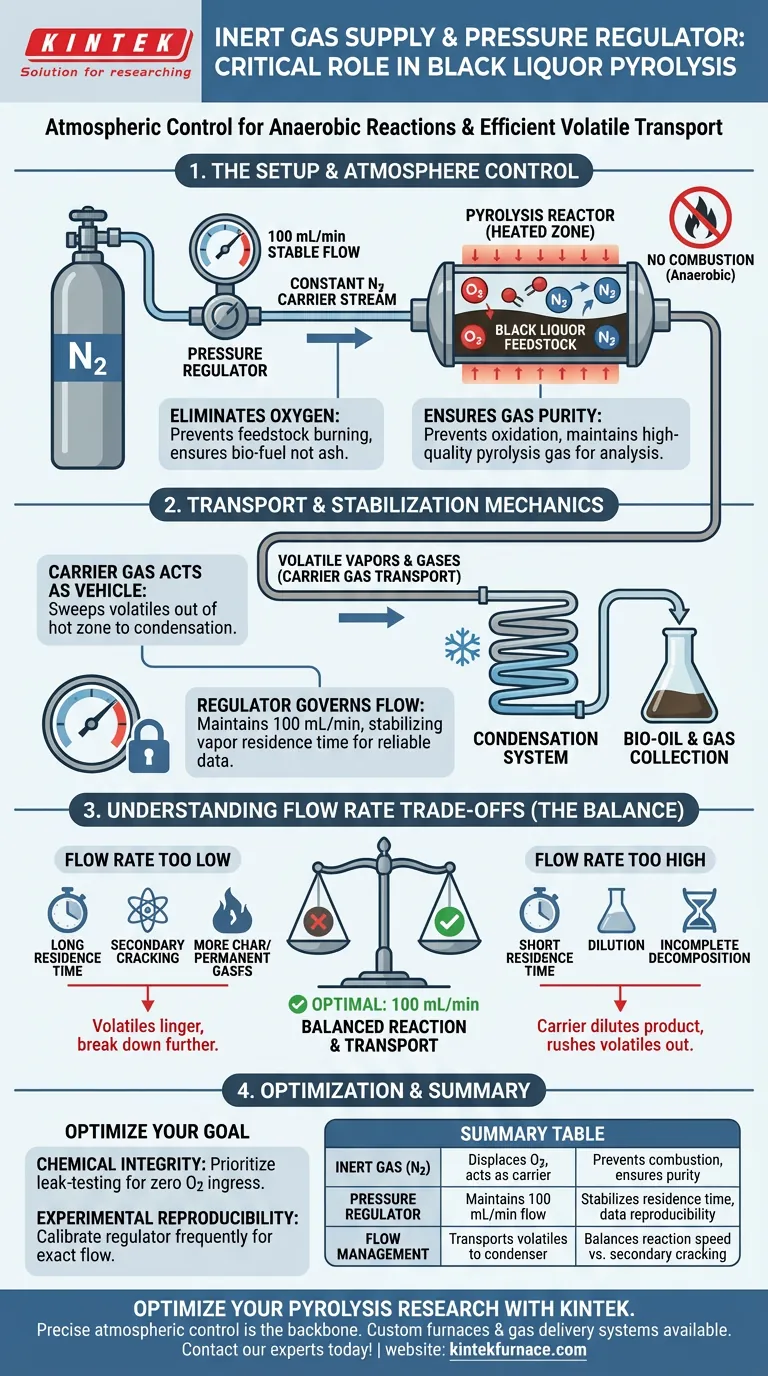
The inert gas supply system serves as the critical atmospheric control unit within a black liquor pyrolysis laboratory setup. It utilizes a precision pressure regulator to deliver a constant, stable flow of Nitrogen (N2) at exactly 100 mL/min, ensuring the reactor environment remains strictly anaerobic throughout the experiment.
By effectively displacing oxygen and maintaining a steady carrier stream, this system prevents the feedstock from combusting and ensures that volatile compounds are efficiently transported to the condensation system for collection.

Creating the Essential Reaction Environment
Preventing Combustion
Pyrolysis is fundamentally defined as thermal decomposition in the complete absence of oxygen.
The primary role of the nitrogen supply is to purge the reactor of all atmospheric air. Eliminating oxygen prevents the black liquor from burning (combustion) at high temperatures, which would result in ash rather than the desired bio-fuels.
Ensuring Gas Purity
Beyond safety, the chemical composition of the output gas is paramount.
The inert N2 environment prevents oxidation reactions that would contaminate the final product. This ensures the purity of the produced pyrolysis gas remains high for accurate analysis.
The Mechanics of Transport and Control
Acting as a Carrier Gas
As the black liquor breaks down under heat, it releases volatile vapors and gases.
The nitrogen stream acts as a vehicle, physically acting as a carrier gas to sweep these volatiles out of the hot reactor zone. It transports them directly to the condensation system, where they can be captured as bio-oil or collected as gas.
The Role of the Pressure Regulator
To achieve reliable experimental data, the flow of nitrogen cannot fluctuate.
The pressure regulator acts as the governor of the system, maintaining a constant flow rate of 100 mL/min. This consistency is essential for stabilizing the residence time of vapors within the reactor.
Understanding the Trade-offs
While the inert gas system is essential, the flow rate must be carefully balanced.
If the flow rate is too high, the carrier gas can dilute the product gases, making detection and analysis more difficult. It may also rush volatiles out of the reactor too quickly, preventing complete decomposition.
Conversely, if the flow rate is too low, volatiles may linger in the hot zone too long. This can lead to "secondary cracking," where valuable vapors break down further into less useful char or permanent gases.
Making the Right Choice for Your Goal
To ensure your black liquor pyrolysis setup yields valid results, you must prioritize the configuration of your gas delivery system.
- If your primary focus is Chemical Integrity: Prioritize leak-testing the entire supply line to ensure zero oxygen ingress, which guarantees the reaction remains true pyrolysis.
- If your primary focus is Experimental Reproducibility: Calibrate your pressure regulator frequently to ensure the flow remains locked at exactly 100 mL/min across all trial runs.
Precise atmospheric control is the fundamental difference between simply burning waste and generating valuable renewable fuel.
Summary Table:
| Component | Primary Function | Experimental Impact |
|---|---|---|
| Inert Gas (N2) | Displaces oxygen & acts as carrier | Prevents combustion; ensures product purity |
| Pressure Regulator | Maintains stable 100 mL/min flow | Stabilizes residence time & data reproducibility |
| Flow Management | Transports volatiles to condenser | Balances reaction speed vs. secondary cracking |
Optimize Your Pyrolysis Research with KINTEK
Precise atmospheric control is the backbone of successful black liquor pyrolysis. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which can be customized with precision gas delivery units to meet your unique laboratory needs. Whether you are scaling up bio-fuel production or perfecting chemical analysis, our high-temperature furnaces provide the stability and control required for reproducible results.
Ready to upgrade your lab setup? Contact our experts today to discuss your custom thermal processing solution!
Visual Guide
References
- Florian Marin, Anca Maria Zaharioiu. Mesoporous Silica Nanocatalyst-Based Pyrolysis of a By-Product of Paper Manufacturing, Black Liquor. DOI: 10.3390/su16083429
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
People Also Ask
- Why are specific temperatures of 848 K, 898 K, and 948 K selected for the Thermal Oxidation of Ti-6Al-4V ELI alloy?
- Why is high-precision temperature control of the heating base critical during FTO spray pyrolysis? Maximize Film Quality
- What are the material selection considerations for multi-layer coatings? Optimize Your Ceramic Molds for Single Crystals
- How do stirring equipment and temperature-controlled heating stages influence magnetic nanoparticle quality?
- What are the benefits of cold compacting and sintering? Boost Efficiency and Cut Costs in Manufacturing
- What are the applications of sintering furnaces in 3D printing? Unlock High-Strength Parts for Aerospace and More
- What role do RTP or continuous sintering furnaces play in solar cell electrode formation? Optimize Your Firing Process
- How does an industrial high-temperature resistance furnace ensure borosilicate fiber quality? Master Thermal Precision



















