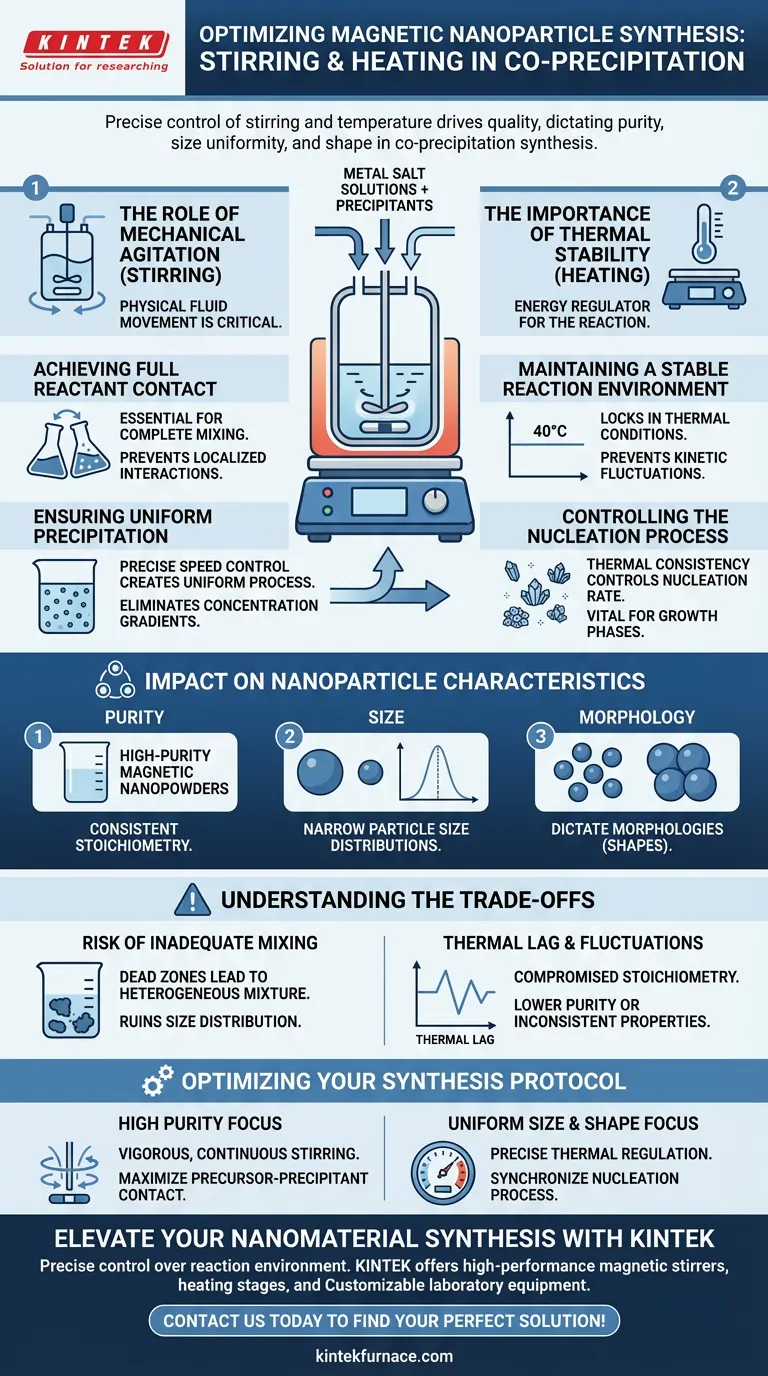
Precise control of stirring and temperature is the fundamental driver of quality in the co-precipitation synthesis of magnetic nanoparticles. These equipment parameters ensure that metal salt solutions and precipitants maintain full contact within a stable reaction environment, directly dictating the purity, size uniformity, and shape of the final material.
Achieving high-quality magnetic nanopowders requires more than just correct chemistry; it demands a homogeneous physical environment. Stirring and heating stages work in tandem to synchronize particle nucleation, ensuring that every nanoparticle forms under identical conditions.
The Role of Mechanical Agitation
The physical movement of fluids within the reactor is as critical as the chemical reagents used.
Achieving Full Reactant Contact
Laboratory stirrers are essential for ensuring that metal salt solutions and precipitants mix completely.
Without continuous mechanical stirring, the interaction between precursors (such as cobalt nitrate) and precipitants (such as ammonia solution) may be localized and inefficient.
Ensuring Uniform Precipitation
By precisely controlling the stirring speed, you create a uniform precipitation process throughout the entire volume of the liquid.
This uniformity prevents concentration gradients, ensuring that all particles experience the same growth environment simultaneously.
The Importance of Thermal Stability
Temperature acts as the energy regulator for the reaction, influencing how quickly particles form and grow.
Maintaining a Stable Reaction Environment
Constant-temperature heating stages, such as magnetic stirring hot plates, are used to lock in specific thermal conditions (e.g., 40°C).
A stable reaction environment is necessary to prevent fluctuations that could alter reaction kinetics unpredictably.
Controlling the Nucleation Process
Thermal consistency is fundamental for controlling the nucleation process, the initial step where the first atoms arrange into a crystalline solid.
Regulating the temperature ensures that nucleation occurs at a controlled rate, which is vital for the subsequent growth phases.
Impact on Nanoparticle Characteristics
The combination of stirring and heating directly dictates the physical and chemical properties of the final product.
Purity and Stoichiometry
Thorough contact and uniform reaction conditions allow for the production of high-purity magnetic nanopowders, such as iron oxide.
This consistency ensures the final product maintains consistent stoichiometry, meaning the ratio of elements in the crystal lattice remains correct throughout the batch.
Size Distribution and Morphology
When the precipitation process is uniform, the resulting nanoparticles exhibit narrow particle size distributions.
Furthermore, these controlled conditions allow researchers to dictate the morphologies (shapes) of the particles, preventing irregular or unwanted structures.
Understanding the Trade-offs
While necessary, the use of this equipment introduces variables that must be carefully managed to avoid diminishing product quality.
The Risk of Inadequate Mixing
If the stirring speed is not optimized for the specific volume and viscosity of the solution, "dead zones" can occur where reactants do not make full contact.
This leads to a heterogeneous mixture where some particles may grow larger than others, ruining the size distribution.
Thermal Lag and Fluctuations
If the heating stage cannot maintain a strictly constant temperature, the stoichiometry of the final product may be compromised.
Fluctuations in heat can disrupt the nucleation process, potentially leading to lower purity or inconsistent magnetic properties.
Optimizing Your Synthesis Protocol
To ensure high-quality magnetic nanoparticles, you must align your equipment settings with your specific material goals.
- If your primary focus is High Purity: Ensure your setup provides vigorous, continuous stirring to maximize the contact between the metal salt precursor and the precipitant.
- If your primary focus is Uniform Size and Shape: Prioritize a heating stage with precise thermal regulation to maintain a stable environment that synchronizes the nucleation process.
Mastering the physical environment of your reaction is the key to unlocking consistent, high-performance nanomaterials.
Summary Table:
| Parameter | Impact on Nanoparticles | Technical Benefit |
|---|---|---|
| Stirring Speed | Eliminates concentration gradients | Ensures narrow particle size distribution |
| Agitation Consistency | Maximizes reactant contact | Promotes high chemical purity and stoichiometry |
| Thermal Stability | Regulates nucleation rate | Prevents irregular morphologies and shapes |
| Temperature Precision | Synchronizes crystal growth | Maintains stable reaction kinetics and quality |
Elevate Your Nanomaterial Synthesis with KINTEK
Precise control over your reaction environment is the difference between inconsistent results and high-purity magnetic nanoparticles. Backed by expert R&D and manufacturing, KINTEK offers high-performance magnetic stirrers, heating stages, and customizable laboratory equipment tailored to your unique synthesis needs.
Whether you are scaling up production or refining delicate morphologies, our systems provide the thermal stability and mechanical precision required for superior material science.
Ready to optimize your lab's performance? Contact us today to find your perfect solution!
Visual Guide
References
- “Pharmaceutical Nanoparticles: Detailed Review of Types, Preparation Methods, and Applications”. DOI: 10.35629/4494-100221922223
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- How does a blast drying oven support the preparation of rubidium-doped mesoporous bioactive glass? Optimized Synthesis
- What is preventive maintenance on a furnace? A Proactive Strategy for Peak Performance
- What happens during the recovery stage of the annealing process? Unlock Stress Relief and Material Restoration
- How does the catalytic steam reforming system convert refinery waste gas into syngas for SOFC? Maximize Waste Energy
- Why is a silicone oil bath preferred for T5 aging of HPDC magnesium alloys? Precision Heat for Peak Strength
- What is the purpose of the annealing process in OLED preparation? Optimize Film Stability and Device Efficiency
- What is the function of magnetron sputtering equipment in Diamond/Cu composites? Enhance Bonding with Precision Coating
- What advantages does a vacuum drying oven offer? Superior Chemical Stability & Efficient Dehydration



















