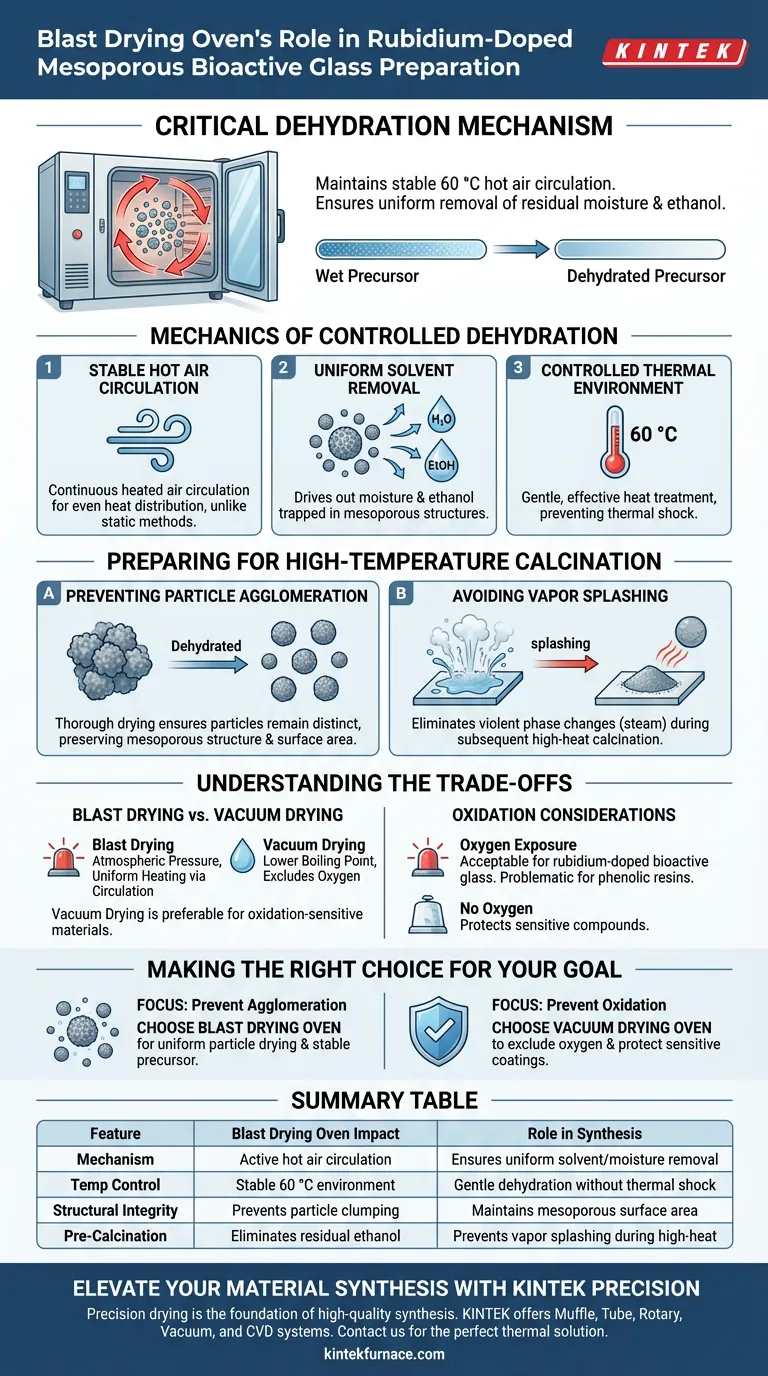
A blast drying oven serves as the critical dehydration mechanism in the synthesis of rubidium-doped mesoporous bioactive glass. By maintaining a stable hot air circulation environment at 60 °C, it ensures the uniform removal of residual moisture and ethanol from washed nanoparticles, creating a thoroughly dried precursor.
The primary function of the blast drying oven is to achieve a completely dehydrated state through active air circulation. This step is a mandatory prerequisite for calcination, ensuring that particles do not agglomerate or suffer from water vapor splashing when later exposed to high temperatures.
The Mechanics of Controlled Dehydration
Stable Hot Air Circulation
The defining feature of a blast drying oven is its ability to circulate heated air continuously. Unlike static drying methods, this circulation ensures that heat is distributed evenly throughout the chamber.
Uniform Solvent Removal
This consistent airflow targets the residual solvents left over from the washing phase. Specifically, it drives out moisture and ethanol trapped within the mesoporous structure of the nanoparticles.
Controlled Thermal Environment
The process operates at a moderate temperature of 60 °C. This provides a gentle yet effective heat treatment that dries the material over the long term without subjecting it to thermal shock before it is ready.
Preparing for High-Temperature Calcination
Preventing Particle Agglomeration
The most significant role of the blast drying oven is preparing the material's physical structure for the next stage. If moisture remains during high-temperature calcination, particles often stick together.
Thorough drying ensures the nanoparticles remain distinct, preserving the mesoporous structure and surface area required for the material's bioactivity.
Avoiding Vapor Splashing
Rapid heating of wet materials can cause trapped liquid to turn into steam instantly, leading to "splashing" or structural rupture.
By achieving a completely dehydrated state in the blast oven first, you eliminate the risk of violent phase changes during the subsequent high-heat calcination process.
Understanding the Trade-offs
Blast Drying vs. Vacuum Drying
While the blast drying oven excels at uniform heating via circulation, it operates at atmospheric pressure.
In contrast, a vacuum drying oven lowers the boiling point of solvents and excludes oxygen. This makes vacuum drying preferable for materials sensitive to oxidation (like MXene nanosheets) or those requiring solvent removal at even lower temperatures to protect delicate frameworks (like pBN-CTF).
Oxidation Considerations
Because a blast drying oven circulates hot air, the material is exposed to oxygen throughout the process.
For rubidium-doped bioactive glass, this is generally acceptable. However, for precursors containing phenolic resins or easily oxidized components, the oxygen-rich environment of a blast oven could compromise the chemical composition.
Making the Right Choice for Your Goal
To ensure synthesis success, align your equipment choice with your specific material constraints:
- If your primary focus is preventing agglomeration: Prioritize the blast drying oven for its ability to uniformly dry particles through active circulation, ensuring a stable precursor for calcination.
- If your primary focus is preventing oxidation: Consider a vacuum drying oven to exclude oxygen and protect sensitive surface coatings or reactive nanosheets.
The blast drying oven acts as the essential stabilization bridge between wet chemical synthesis and the high-temperature formation of the final bioactive glass structure.
Summary Table:
| Feature | Blast Drying Oven Impact | Role in Synthesis |
|---|---|---|
| Mechanism | Active hot air circulation | Ensures uniform solvent/moisture removal |
| Temp Control | Stable 60 °C environment | Gentle dehydration without thermal shock |
| Structural Integrity | Prevents particle clumping | Maintains mesoporous surface area for bioactivity |
| Pre-Calcination | Eliminates residual ethanol | Prevents vapor splashing during high-heat stages |
Elevate Your Material Synthesis with KINTEK Precision
Precision drying is the foundation of high-quality bioactive glass and nanoparticle synthesis. At KINTEK, we understand that the transition from wet chemistry to calcination requires exact thermal control and uniformity.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside specialized lab drying solutions. Our high-temperature furnaces and ovens are fully customizable to meet your unique research or production requirements, ensuring your mesoporous structures remain intact and agglomeration-free.
Ready to optimize your dehydration and calcination workflows? Contact us today to find the perfect thermal solution for your lab!
Visual Guide
References
- Usanee Pantulap, Aldo R. Boccaccini. Hydroxycarbonate apatite formation, cytotoxicity, and antibacterial properties of rubidium-doped mesoporous bioactive glass nanoparticles. DOI: 10.1007/s10934-023-01546-9
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What is the function of high-purity nitrogen (N2) during the heating phase of magnetite oxidation? Protect Your Data.
- Why is the vacuum drying process essential for the synthesis of phthalonitrile-modified titanium dioxide? Expert Guide
- What is the role of a TG-FTIR-MS coupled system in 5AT and NaIO4 analysis? Master Thermal Decomposition Insights
- What are the functions of hydrogen gas for graphene on silver? Enhance Crystallinity & Stability
- What type of reaction environment is required for the synthesis of Ge-Se-Tl-Sb chalcogenide glasses? | KINTEK
- Why is uniform heating important in industrial processes? Ensure Quality and Efficiency in Manufacturing
- Why is 5G network infrastructure critical for real-time quality control? Achieve Zero-Defect Thermal Processing
- How do lab furnaces simulate fire environments for UHPFRC testing? Achieving ISO834 Standard Compliance



















