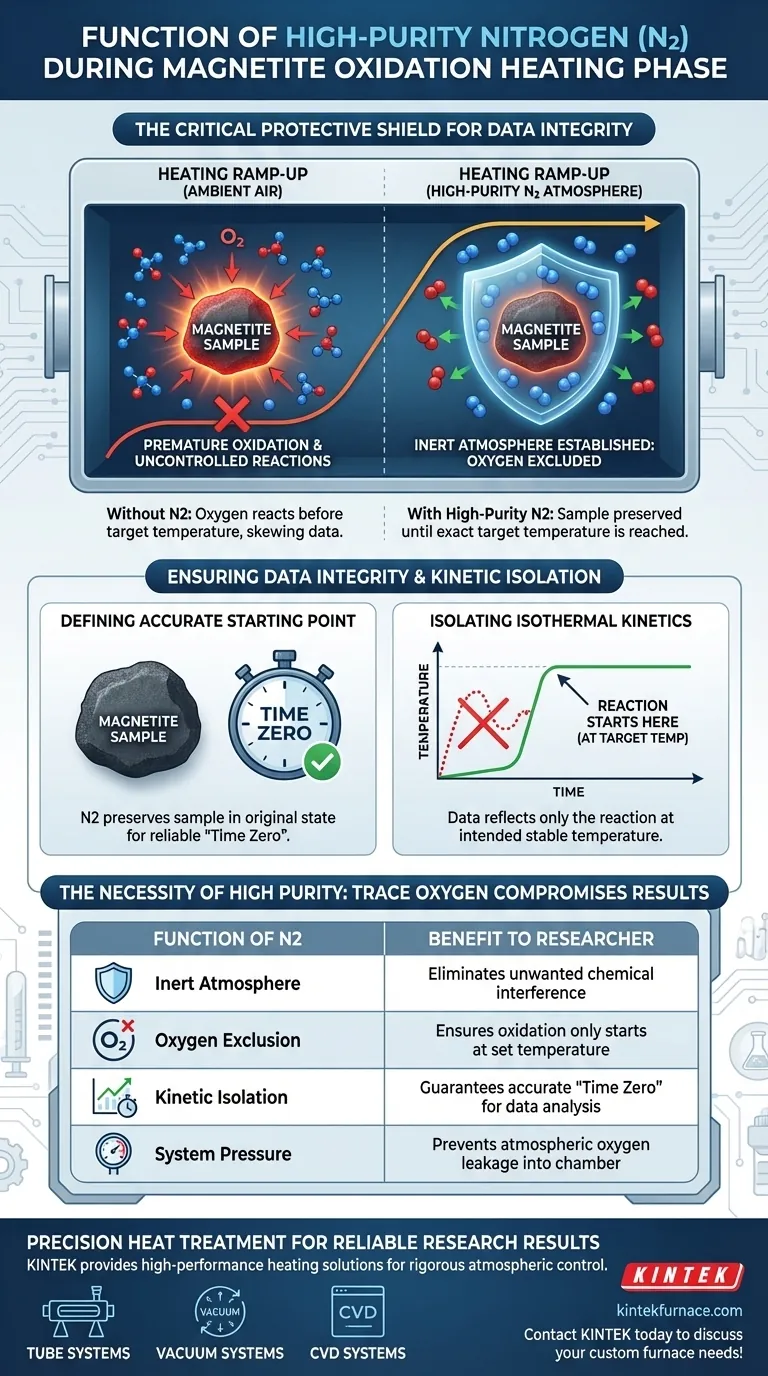
High-purity nitrogen (N2) acts as a critical protective shield during the heating phase of magnetite oxidation experiments. Its primary function is to create an inert atmosphere that completely excludes oxygen from the reaction chamber while the sample temperature is ramped up to the target level.
The use of high-purity nitrogen prevents uncontrolled oxidation before the experiment officially begins, ensuring that all kinetic data reflects the reaction at the specific target temperature rather than the heating process.

Creating a Controlled Environment
Establishing an Inert Atmosphere
The introduction of high-purity nitrogen is essential for displacing reactive gases within the experimental system. By filling the chamber with N2, you create an environment where chemical reactions are effectively paused. This is vital because nitrogen is chemically inert relative to magnetite under these specific conditions.
Excluding Oxygen
The most critical function of this nitrogen purge is the total exclusion of oxygen. Magnetite is highly reactive with oxygen, especially as temperatures rise. Without a nitrogen blanket, oxygen from the ambient air would immediately begin reacting with the sample as soon as heating commences.
Preventing Premature Oxidation
If oxygen were present during the heating phase, the magnetite would begin to oxidize before reaching the set isothermal temperature (e.g., 973 K or 1073 K). This "premature" oxidation is uncontrolled and occurs at a range of increasing temperatures, rather than the specific temperature you intend to study.
Ensuring Data Integrity
Defining an Accurate Starting Point
For kinetic data to be valid, the starting point of the reaction must be clearly defined. High-purity nitrogen preserves the magnetite in its original state until the exact moment the target temperature is reached. This ensures that "Time Zero" of your experiment corresponds to a fresh, unreacted sample.
Isolating Isothermal Kinetics
The goal of these experiments is often to understand oxidation kinetics at a specific, constant temperature (isothermal). If the sample partially reacts during the heating ramp-up, the resulting data becomes a mixture of non-isothermal and isothermal reactions. Nitrogen ensures the data reflects only the reaction at the intended stable temperature.
Understanding the Trade-offs
The Necessity of High Purity
It is not enough to simply use nitrogen; the gas must be high-purity. If standard commercial nitrogen containing trace amounts of oxygen is used, the "protective" atmosphere is compromised. Even small amounts of oxygen can induce surface oxidation during the heating ramp, subtly skewing the starting baseline of your data.
System Sealing
The effectiveness of the nitrogen atmosphere relies heavily on the integrity of the system. A continuous flow of high-purity nitrogen is required to maintain positive pressure and prevent ambient air from leaking back into the heating chamber.
Making the Right Choice for Your Goal
To ensure the validity of your magnetite oxidation studies, consider your specific experimental objectives:
- If your primary focus is determining accurate reaction rates: Ensure the nitrogen flow is established well before heating begins to flush all oxygen from the system.
- If your primary focus is analyzing the final oxidation product: Use high-purity nitrogen to guarantee that the structural changes observed are a result of the target temperature, not the heating ramp.
By rigorously controlling the atmosphere during heating, you transform variable, messy data into precise, scientific insight.
Summary Table:
| Function of N2 | Purpose in Experiment | Benefit to Researcher |
|---|---|---|
| Inert Atmosphere | Displaces reactive gases/ambient air | Eliminates unwanted chemical interference |
| Oxygen Exclusion | Prevents pre-reaction during ramp-up | Ensures oxidation only starts at the set temperature |
| Kinetic Isolation | Maintains sample in its original state | Guarantees accurate "Time Zero" for data analysis |
| System Pressure | Maintains positive pressure flow | Prevents atmospheric oxygen leakage into chamber |
Precision Heat Treatment for Reliable Research Results
Don't let uncontrolled oxidation compromise your experimental integrity. KINTEK provides high-performance heating solutions—including Tube, Vacuum, and CVD systems—specifically designed to maintain high-purity atmospheres with rigorous sealing.
Backed by expert R&D and world-class manufacturing, we offer customizable lab high-temp furnaces to meet the unique demands of your magnetite studies and material science research. Ensure your data reflects the truth of your experiments.
Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- A. Laarich, Kurt N. Wiegel. Effect of Particle Size on Magnetite Oxidation Behavior: A Modeling Approach Incorporating Ultra-Fine Particle Effects. DOI: 10.1007/s11663-025-03640-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- Why is pre-sintering of Ga2O3 raw material powder required? Unlock Beta-Phase Stability for High-Performance Thin Films
- Why is rapid water quenching necessary after thermal compression? Capture True Microstructures in Medium-Mn Steel
- What functions do high-strength graphite molds perform during SPS? Drive Efficiency & Precision in Material Bonding
- Why is the use of high-temperature furnace systems critical for delta-MnO2 development? Master Atomic Engineering
- Why is High-Temperature Annealing Required for WS2 Gas Sensors? Stabilize Performance & Eliminate Drift
- What are the advantages of using a nitrate salt bath furnace? Superior Quenching for Sorbitic Steel Wire
- What is the design logic behind the double-layer reactor structure used in the ITSP process? Optimize Your Fuel Quality
- What is the role of temperature control in MCM-41 synthesis? Master Precision Pore Engineering



















