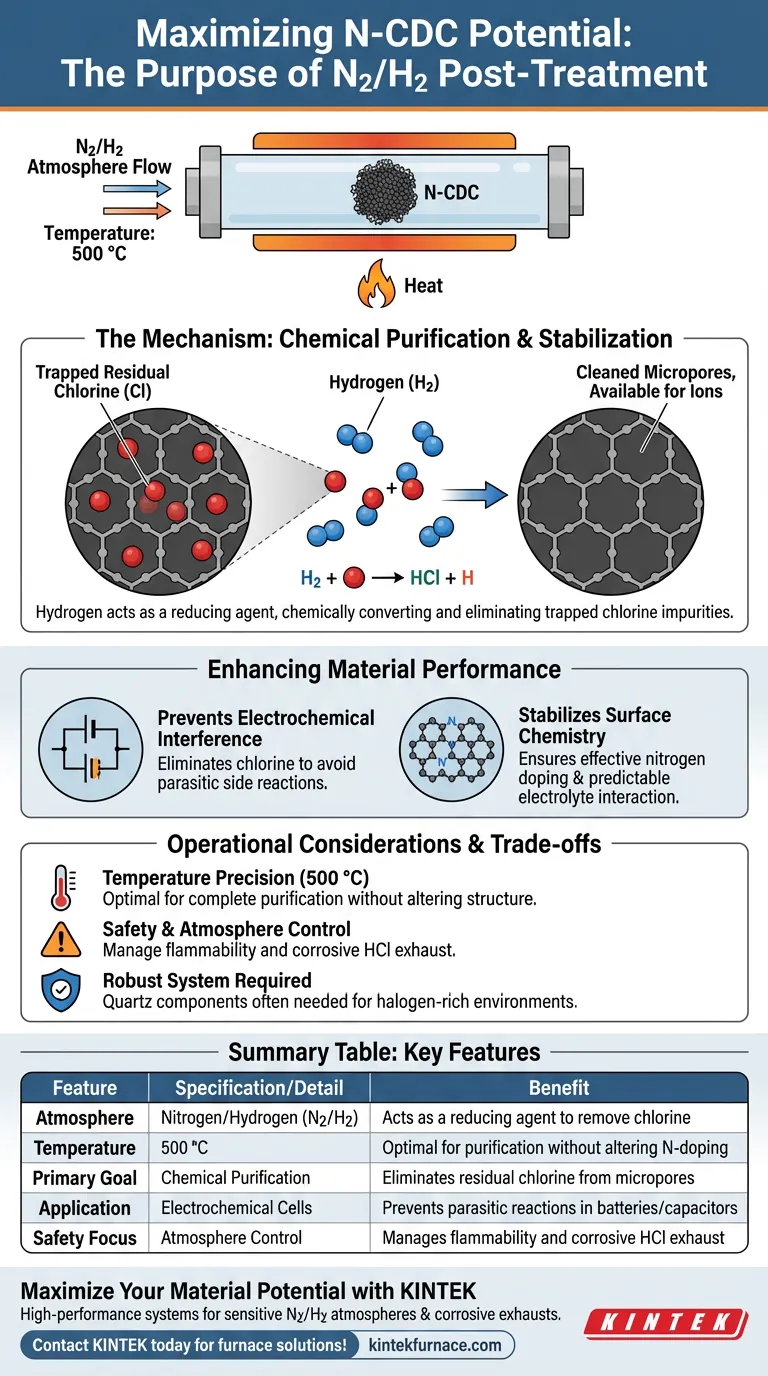
The primary purpose of post-treating Nitrogen-doped Carbide-Derived Carbon (N-CDC) in a nitrogen and hydrogen (N2/H2) atmosphere is to purify and stabilize the carbon structure.
By heating the material to 500 °C within a tube furnace, the process leverages the reducing properties of hydrogen. This effectively eliminates residual chlorine atoms that are trapped within the material's microporous structure during previous synthesis steps.
Core Takeaway This post-treatment is effectively a chemical purification step, not just a thermal one. By introducing hydrogen, you actively convert and remove trapped chlorine impurities, which is essential for ensuring the material’s stability and preventing interference during electrochemical applications.

The Mechanism of Purification
Leveraging Hydrogen Reduction
The presence of hydrogen (H2) in the atmosphere is the critical factor in this process.
While heat provides the energy, hydrogen acts as a reducing agent. It chemically reacts with the residual chlorine species remaining from the initial etching process.
Clearing the Microporous Structure
Carbide-Derived Carbon is known for its complex, microporous network.
During the synthesis phase—often involving chlorination etching—chlorine atoms can become physically or chemically trapped deep within these pores. The N2/H2 treatment flushes these atoms out, ensuring the pore volume is available for ions rather than being clogged by synthesis byproducts.
Enhancing Material Performance
Preventing Electrochemical Interference
The most significant risk of skipping this step is the presence of residual chlorine in the final product.
Chlorine is chemically active and can cause unwanted side reactions in electrochemical cells. By removing it, you prevent these parasitic reactions that would otherwise degrade the performance of supercapacitors or batteries utilizing the N-CDC.
Stabilizing Surface Chemistry
Beyond just removing impurities, this treatment acts as a final stabilization step for the carbon framework.
The reducing atmosphere helps to settle the surface chemical state of the carbon. This ensures that the nitrogen doping remains effective and that the carbon surface interacts predictably with electrolytes.
Operational Considerations and Trade-offs
Temperature Precision is Critical
The process requires a specific temperature target of 500 °C to be effective.
Temperatures significantly lower than this may fail to fully activate the hydrogen reduction, leaving residual chlorine behind. Conversely, excessive temperatures could potentially alter the desired nitrogen-doping levels or carbon structure.
Safety and Atmosphere Control
Using hydrogen, even in a mixture, requires strict adherence to safety protocols due to flammability.
Furthermore, because the process releases chlorine-based compounds (likely HCl gas), the tube furnace system must be robust. As noted in general processing standards, materials like quartz are often required to withstand the high-temperature corrosive nature of halogen-rich environments.
Making the Right Choice for Your Goal
To maximize the potential of your N-CDC material, ensure your post-treatment protocols are strictly defined.
- If your primary focus is Electrochemical Stability: Ensure the process reaches a full 500 °C to guarantee the complete removal of chlorine, which is the main source of interference.
- If your primary focus is Material Purity: Monitor the exhaust of the tube furnace; the cessation of acidic byproducts indicates that the hydrogen has successfully purged the micropores.
Success in N-CDC synthesis relies not just on creating the pores, but on rigorously cleaning them to unlock the material's full potential.
Summary Table:
| Feature | Specification/Detail | Benefit |
|---|---|---|
| Atmosphere | Nitrogen/Hydrogen (N2/H2) | Acts as a reducing agent to remove chlorine |
| Temperature | 500 °C | Optimal for purification without altering N-doping |
| Primary Goal | Chemical Purification | Eliminates residual chlorine from micropores |
| Application | Electrochemical Cells | Prevents parasitic reactions in batteries/capacitors |
| Safety Focus | Atmosphere Control | Manages flammability and corrosive HCl exhaust |
Maximize Your Material Potential with KINTEK
Precision is non-negotiable when purifying Nitrogen-doped Carbide-Derived Carbon. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Tube, Muffle, Vacuum, and CVD systems designed to handle sensitive N2/H2 atmospheres and corrosive exhausts. Whether you need standard lab high-temp furnaces or a fully customizable solution tailored to your unique synthesis needs, our engineering team is ready to help you achieve superior results.
Ready to elevate your research? Contact KINTEK today to find the perfect furnace for your electrochemical applications!
Visual Guide
References
- Berta Pérez‐Román, Fernando Rubio‐Marcos. Synergistic Effect of Nitrogen Doping and Textural Design on Metal-Free Carbide-Derived Carbon Electrocatalysts for the ORR. DOI: 10.1021/acsami.5c10307
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- How do industrial heat treatment furnaces ensure 55Si2 spring steel stability? Optimize Your Tempering Process
- What are the functions of a programmed temperature rise experimental system? Master Coal Pre-Oxidation Research
- How does a circulating cooling water system contribute to the removal of impurities? Optimize Rubidium Chloride Purity
- Why use a precision oven for moxa floss samples? Ensure Accurate Air-Drying Basis for Combustion Research
- What is quenching, and why is it important? Achieve Superior Material Hardness and Strength
- Why must Ba1-xCaxTiO3 ceramic samples undergo a high-temperature silver-firing process? Ensure Accurate Dielectric Data
- Why is constant temperature heating required for HfC precursors? Master HfOC/SiOC Composite Pre-treatment
- What are the core process advantages of using a microwave reactor? Maximize Speed & Efficiency in Lab Characterization



















