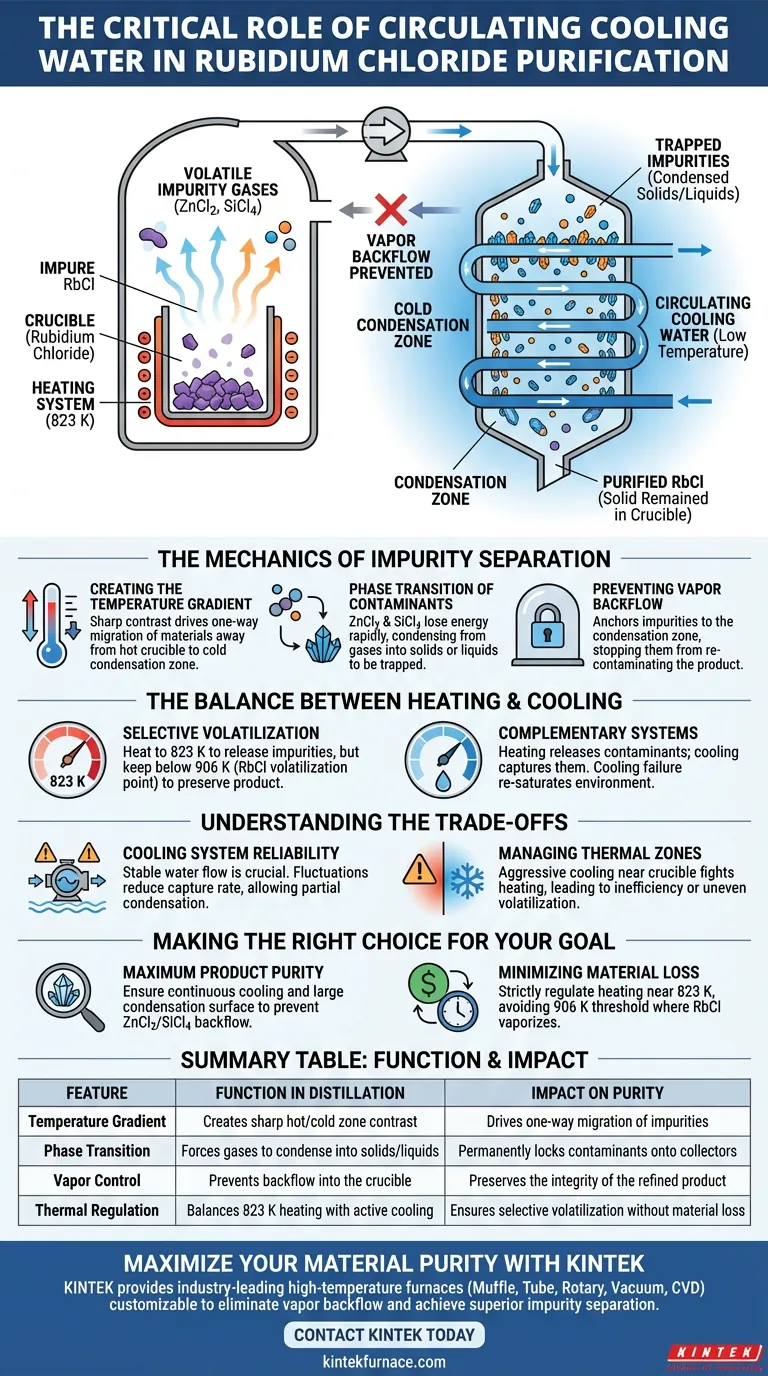
The circulating cooling water system functions as the critical mechanism for permanently trapping volatile impurities. By circulating through condensation collection devices, it creates a sharp temperature gradient that rapidly cools volatilized impurity gases like Zinc Chloride (ZnCl2) and Silicon Tetrachloride (SiCl4). This forces these contaminants to undergo a phase change from gas back into solids or liquids, effectively capturing them before they can re-contaminate the Rubidium Chloride.
In a vacuum distillation setup, heating releases the impurities, but cooling determines the final purity. The cooling water system ensures that once impurities are vaporized, they are solidified in a separate zone, preventing vapor backflow and preserving the integrity of the product in the crucible.

The Mechanics of Impurity Separation
Creating the Temperature Gradient
The purification process relies on a stark contrast in temperatures. While the furnace heats the material to release gases, the circulating cooling water maintains a specific zone at a much lower temperature.
This temperature gradient is the engine that drives the physical separation of materials. It ensures that migration is one-way: away from the hot crucible and toward the cold condensation zone.
Phase Transition of Contaminants
As impurity gases such as ZnCl2 and SiCl4 contact the surfaces cooled by the water system, they lose thermal energy instantly.
This rapid energy loss forces the gases to condense into liquids or deposit as solids. By changing the state of the matter, the cooling system effectively "locks" the impurities onto the collection device.
Preventing Vapor Backflow
Without active cooling, volatile gases would remain in a vapor state within the vacuum chamber.
If these gases remain suspended, they pose a risk of vapor backflow, where they drift back into the crucible. The cooling water system mitigates this by anchoring the impurities to the condensation zone, ensuring they cannot return to contaminate the purified Rubidium Chloride.
The Balance Between Heating and Cooling
Selective Volatilization
To understand the cooling system's value, one must understand the heating strategy. The system is heated to approximately 823 K, a temperature sufficient for the kinetic decomposition and volatilization of impurities.
Critically, this is kept below 906 K (the volatilization point of Rubidium Chloride at 5 Pa). This ensures that only the impurities become gases that the cooling system needs to manage, while the Rubidium Chloride remains solid.
Complementary Systems
The heating system creates the separation potential, but the cooling system executes the capture.
If the cooling water fails to maintain the gradient, the precise thermal control at 823 K becomes irrelevant because the liberated impurities will simply re-saturate the environment.
Understanding the Trade-offs
Cooling System Reliability
The efficiency of impurity removal is directly tied to the stability of the circulating water.
Fluctuations in water flow or temperature can reduce the capture rate of the condensation devices. If the "trap" is not cold enough, partial condensation may occur, allowing lighter gases to remain in the vacuum stream.
Managing Thermal Zones
There is a delicate balance in system design between the hot zone and the cold zone.
If the cooling effect is too aggressive or poorly insulated from the crucible, it may fight against the heating elements. This can lead to energy inefficiency or uneven heating of the Rubidium Chloride, potentially impacting the volatilization rate of the impurities.
Making the Right Choice for Your Goal
To maximize the effectiveness of your vacuum distillation setup, align your operational focus with your specific objectives:
- If your primary focus is maximum product purity: Ensure the cooling water flow is continuous and the condensation surface area is maximized to prevent any vapor backflow of ZnCl2 or SiCl4.
- If your primary focus is minimizing raw material loss: Strictly monitor the heating regulation to stay near 823 K, ensuring you do not exceed the 906 K threshold where Rubidium Chloride begins to vaporize.
Successful purification requires the precise synchronization of controlled heating to release contaminants and aggressive cooling to capture them.
Summary Table:
| Feature | Function in Distillation | Impact on Purity |
|---|---|---|
| Temperature Gradient | Creates a sharp contrast between hot and cold zones | Drives one-way migration of impurities |
| Phase Transition | Forces gases to condense into liquids or solids | Permanently locks contaminants onto collectors |
| Vapor Control | Prevents backflow into the crucible | Preserves the integrity of the refined product |
| Thermal Regulation | Balances 823 K heating with active cooling | Ensures selective volatilization without material loss |
Maximize Your Material Purity with KINTEK
Precision in vacuum distillation requires the perfect balance of heating and cooling. KINTEK provides industry-leading laboratory high-temperature furnaces, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet your specific research or production needs. Backed by expert R&D and manufacturing, we help you eliminate vapor backflow and achieve superior impurity separation.
Ready to upgrade your thermal processing setup? Contact KINTEK Today to discuss your custom solution with our specialists.
Visual Guide
References
- Cui Xi, Tao Qu. A Study on the Removal of Impurity Elements Silicon and Zinc from Rubidium Chloride by Vacuum Distillation. DOI: 10.3390/ma17091960
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- Why is high-precision temperature control at 800 °C critical for BCMoMn catalyst heterostructures?
- What role does phosphoric acid (H3PO4) play during the chemical activation stage of sawdust? Boost Porosity & Efficiency
- What is the role of high-temperature calcination equipment in Waste Tire Charcoal preparation? Master WTC Pyrolysis
- What is the function of an electric arc furnace in the preparation of aluminum-silicon model alloys? Expert Insights
- Why is a final drying step necessary when restructuring adsorbents? Ensure Chemical Bonding & Industrial Safety
- What is the objective of setting temperature gradients of 40 °C, 50 °C, and 60 °C? Optimize Yogurt Drying Viability
- What is the purpose of using a thermal evaporation coating system? Enhancing I-V Testing Accuracy for Nanocomposites
- What are the key characteristics of furnaces used in 3D printing sintering? Achieve Precision Sintering for High-Quality Parts



















