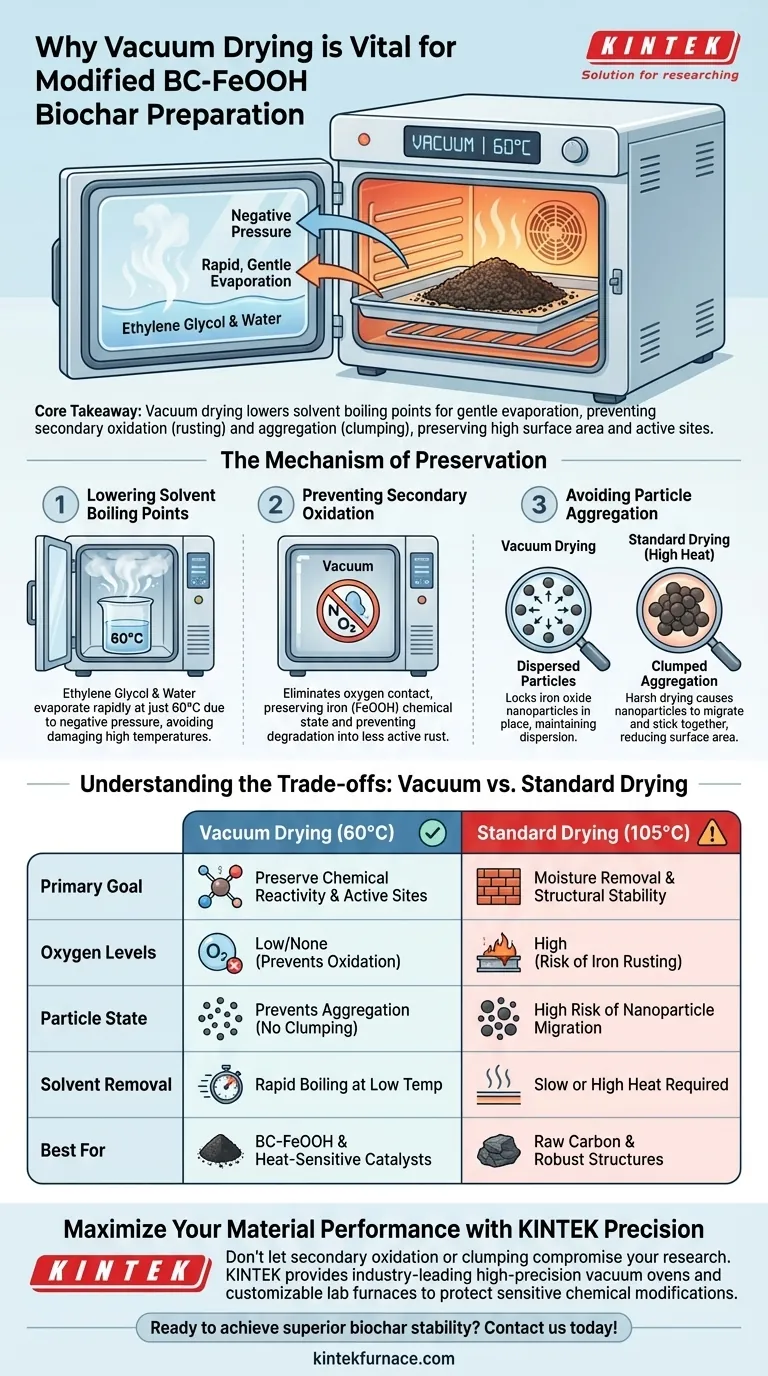
The use of a vacuum drying oven is a precise synthesis step designed to protect the chemical reactivity of modified BC-FeOOH biochar. By operating at a controlled 60°C under negative pressure, this equipment allows for the rapid removal of solvents—specifically residual ethylene glycol and water—without subjecting the material to damaging high temperatures.
Core Takeaway: Vacuum drying lowers the boiling point of solvents to enable gentle evaporation. This prevents the iron oxides from rusting (secondary oxidation) or clumping (aggregation), ensuring the biochar retains the high surface area and active sites necessary for its intended performance.

The Mechanism of Preservation
To understand why standard drying methods fail for this specific material, one must look at the interaction between pressure, temperature, and chemical stability.
Lowering Solvent Boiling Points
The primary challenge in this preparation is removing ethylene glycol and water. Ethylene glycol typically has a high boiling point, making it difficult to remove in a standard oven without cranking up the heat.
Under the negative pressure of a vacuum oven, the boiling points of these liquids drop significantly. This allows them to evaporate rapidly at just 60°C, a temperature that is safe for the biochar's structure.
Preventing Secondary Oxidation
Iron oxides (FeOOH) are chemically active and sensitive to their environment. If exposed to high heat in the presence of air, they are prone to secondary oxidation.
Vacuum drying removes oxygen from the chamber. By eliminating air contact during the heating process, the specific chemical state of the iron is preserved, preventing it from degrading into less active forms of rust.
Avoiding Particle Aggregation
Performance relies on the microscopic distribution of active particles. If drying is too harsh or slow, nanoparticles tend to migrate and stick together.
This "clumping" phenomenon, known as aggregation, drastically reduces the material's surface area. The gentle, rapid vacuum drying locks the iron oxide particles in place, maintaining their dispersion across the biochar surface.
Understanding the Trade-offs
While vacuum drying is superior for chemically sensitive modifications like BC-FeOOH, it is important to understand when and why other methods are used.
Vacuum vs. Standard Constant Temperature
Standard constant temperature ovens (often set to 105°C) are typically used for removing moisture from raw materials or robust carbon structures, such as chitin-derived carbon.
In those cases, the goal is simply to prevent capillary forces from collapsing the pore structure.
The Cost of Complexity
Vacuum drying is more complex and equipment-intensive than standard drying. However, for BC-FeOOH, using a standard oven would likely result in oxidized, clumped iron particles, rendering the biochar ineffective despite a preserved pore structure.
Making the Right Choice for Your Goal
The choice of drying method dictates the final quality of your catalytic or adsorbent material.
- If your primary focus is preserving chemically active sites (e.g., metal oxides): Use vacuum drying to lower oxidation risks and prevent particle clumping at low temperatures.
- If your primary focus is structural stability of raw carbon: Use a standard constant temperature oven to thoroughly remove moisture and prevent pore collapse due to capillary forces.
In biochar modification, the drying step is not just about removing water; it is about freezing the material's chemistry in its most effective state.
Summary Table:
| Feature | Vacuum Drying (60°C) | Standard Drying (105°C) |
|---|---|---|
| Primary Goal | Preserve chemical reactivity & active sites | Moisture removal & structural stability |
| Oxygen Levels | Low/None (Prevents secondary oxidation) | High (Risk of iron rusting) |
| Particle State | Prevents aggregation (clumping) | High risk of nanoparticle migration |
| Solvent Removal | Rapid boiling at low temperature | Slow evaporation or requires high heat |
| Best For | BC-FeOOH and heat-sensitive catalysts | Raw carbon materials and robust structures |
Maximize Your Material Performance with KINTEK Precision
Don't let secondary oxidation or particle clumping compromise your research. KINTEK provides industry-leading thermal solutions, including high-precision vacuum ovens and customizable lab furnaces, designed to protect your most sensitive chemical modifications. Backed by expert R&D and manufacturing, our range includes Muffle, Tube, Rotary, and Vacuum systems tailored for your unique synthesis needs.
Ready to achieve superior biochar stability? Contact us today to find the perfect drying solution for your lab!
Visual Guide
References
- Yong Dai, Ruyi Zheng. Adsorption and removal of pentavalent antimony from water by biochar prepared from modified rosa roxburghii residue. DOI: 10.3389/fenvs.2024.1540638
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is 500°C thermal stabilization necessary for titania supports? Ensure Catalyst Stability and Performance
- What are batch catalytic debinding ovens used for? Speed Up MIM/CIM with Low-Temp Debinding
- What are the advantages of electric current-assisted TLP bonding? Maximize Efficiency for Inconel 718 Joining
- What are the advantages of using an acid oxidation bath? Accelerate Lignin Fiber Stabilization from Hours to Minutes
- What is the primary role of the Thermal Oxidation (TO) process in Ti-6Al-4V ELI alloy? Enhancing Hardness and Wear
- What are the advantages of SLRP compared to traditional high-temperature furnaces? Revolutionizing UHTC Coatings
- What role does an industrial-grade POCl3 diffusion furnace system play in DOSS? Master Quantitative Phosphorus Control
- How does the design of specialized industrial furnaces for hydrogen production contribute to extension of lifespan?



















