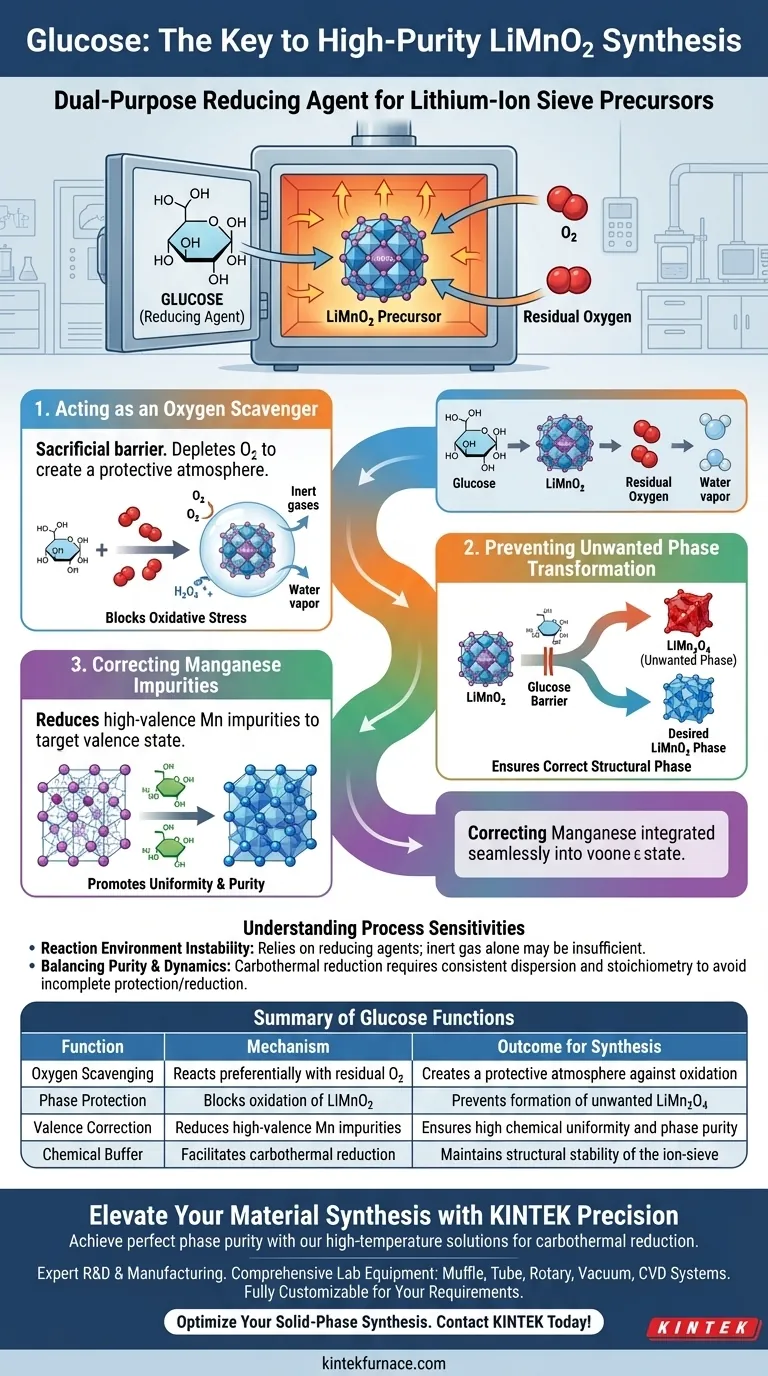
Glucose functions primarily as a dual-purpose reducing agent essential for maintaining the chemical integrity of the precursor during synthesis. In high-temperature solid-phase reactions, it acts as a sacrificial barrier against residual oxygen and actively corrects the valence state of manganese to ensure the formation of high-purity LiMnO2.
The central value of glucose lies in its ability to facilitate carbothermal reduction, protecting the target material from oxidation. Without this intervention, the synthesis process is liable to produce unwanted LiMn2O4 rather than the desired LiMnO2 precursor.

The Mechanics of Carbothermal Reduction
Acting as an Oxygen Scavenger
In high-temperature environments, residual oxygen poses a significant threat to the stability of the synthesis. Glucose serves as a sacrificial reducing agent.
It reacts preferentially with oxygen in the reaction environment, effectively "taking the hit" to deplete oxygen levels. This process creates a protective atmosphere that shields the developing material from oxidative stress.
Preventing Unwanted Phase Transformation
The primary objective of this protection is to preserve the LiMnO2 phase.
If exposed to excess oxygen without the presence of glucose, LiMnO2 is prone to oxidizing into LiMn2O4. By consuming the oxygen, glucose blocks this chemical pathway, ensuring the material remains in the correct structural phase.
Correcting Manganese Impurities
Beyond atmospheric protection, glucose plays an active role in the internal chemistry of the material.
It reduces small amounts of high-valence manganese impurities that may form during the reaction. By returning these impurities to their target valence state, glucose ensures the uniformity and phase purity of the final product.
Understanding the Process Sensitivities
The Dependency on Sacrificial Agents
While glucose is effective, its use highlights the instability of the reaction environment.
The synthesis of LiMnO2 is highly sensitive to oxidation, meaning the process relies heavily on the presence of a reducing agent. This implies that standard inert gas atmospheres alone may be insufficient to guarantee phase purity without chemical assistance.
Balancing Purity and Reaction Dynamics
The carbothermal reduction effect is a powerful tool, but it introduces a variable into the solid-phase synthesis.
The efficiency of the purification depends on the glucose successfully reacting with both the environmental oxygen and the internal impurities. Inconsistent dispersion or stoichiometry could theoretically lead to localized oxidation (incomplete protection) or incomplete reduction of impurities.
Ensuring Synthesis Success
To maximize the quality of your lithium-ion sieve precursors, consider the following strategic applications of glucose:
- If your primary focus is Phase Purity: Ensure sufficient glucose is present to fully consume residual oxygen, preventing the formation of the LiMn2O4 contaminant.
- If your primary focus is Chemical Consistency: Rely on the carbothermal reduction mechanism to standardize the valence state of manganese across the entire sample.
By leveraging glucose as a chemical buffer, you secure the structural stability required for effective ion-sieve performance.
Summary Table:
| Function of Glucose | Mechanism | Outcome for Synthesis |
|---|---|---|
| Oxygen Scavenging | Reacts preferentially with residual O2 | Creates a protective atmosphere against oxidation |
| Phase Protection | Blocks oxidation of LiMnO2 | Prevents formation of unwanted LiMn2O4 |
| Valence Correction | Reduces high-valence Mn impurities | Ensures high chemical uniformity and phase purity |
| Chemical Buffer | Facilitates carbothermal reduction | Maintains structural stability of the ion-sieve |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect phase purity for lithium-ion sieve precursors requires more than just the right chemistry—it demands a controlled thermal environment. KINTEK provides industry-leading high-temperature solutions to master your carbothermal reduction processes.
Backed by expert R&D and manufacturing, we offer a comprehensive range of lab equipment including Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are synthesizing LiMnO2 or advanced battery materials, our furnaces are fully customizable to meet your unique atmospheric and temperature requirements.
Ready to optimize your solid-phase synthesis? Contact KINTEK today to discover how our high-performance heating solutions can ensure your chemical consistency and structural stability.
Visual Guide
References
- Jing Zhu, Run-Min Yao. Synthesis of Porous Lithium Ion Sieve with High Purity for Li+ Adsorption. DOI: 10.3390/ma18102373
This article is also based on technical information from Kintek Furnace Knowledge Base .
People Also Ask
- What is the significance of using different sizes of steel working ampoules? Precision vs. Efficiency in Lab Research
- Why must temperature loss be monitored during the aluminum alloy refining cycle? Essential Tips for Casting Success
- Why is an industrial electric drying oven required for catalyst support precursors? Secure Pore Integrity
- What role does helium play in nanoparticle synthesis? Unlock Precision via Inert Gas Condensation
- What are the advantages of combining vacuum hot rolling with small hole vacuuming? High-Bonding Clad Plate Production
- What core parameters does a sessile drop furnace provide for quartz glass? Master High-Temp Material Evaluation
- How does an autoclave assist in modifying bio-carbon with cobalt oxide? Unlock High-Performance Nano-Composites
- What is the function of a drying oven in the post-treatment process of Ni and Zn-doped MgO nanoparticles?