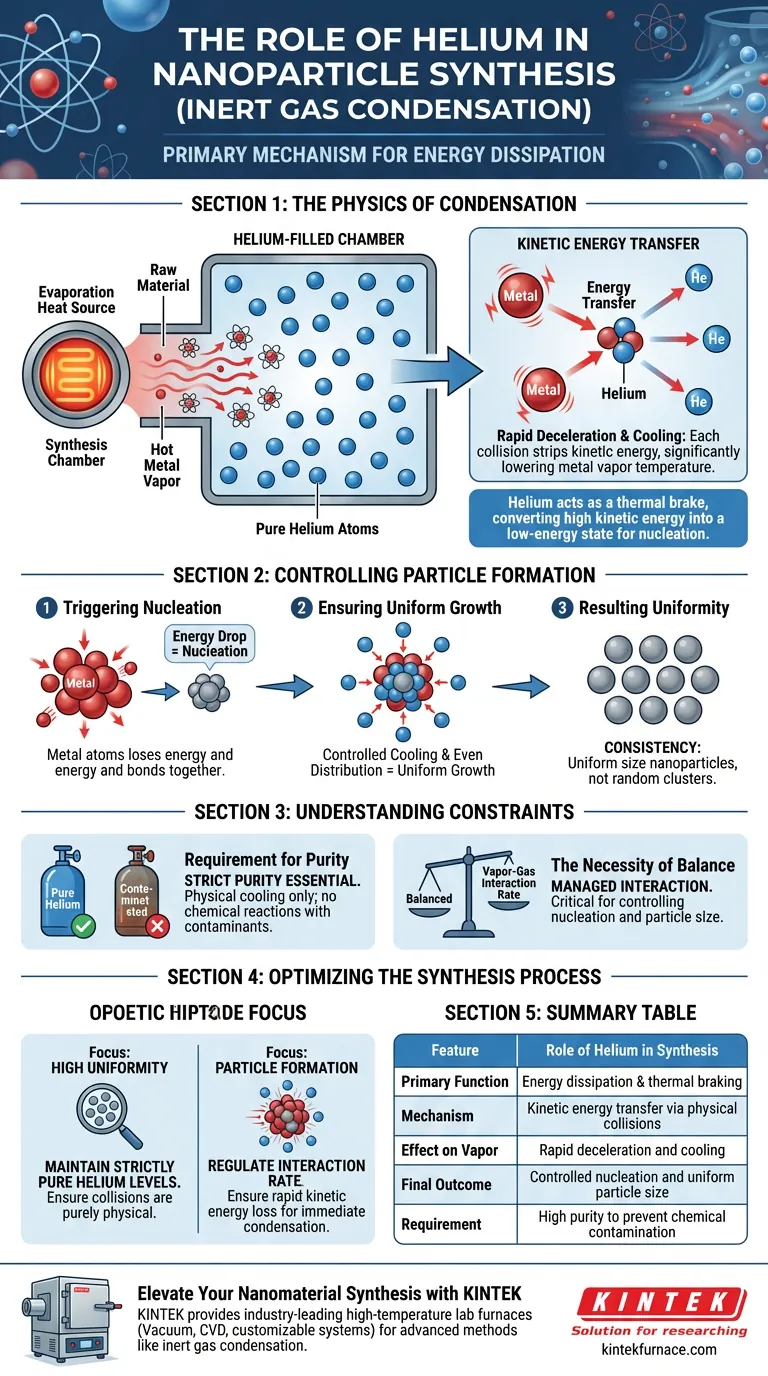
Helium serves as the primary mechanism for energy dissipation in the inert gas condensation method. When raw materials are evaporated into a gas phase, they enter a chamber filled with pure helium, where the gas acts as a direct coolant. Through physical collisions with the hot metal vapor, helium atoms strip away kinetic energy, causing the rapid deceleration and condensation necessary to form solid nanoparticles.
The helium atmosphere acts as a thermal brake, converting the high kinetic energy of metal vapor into the low-energy state required for nucleation. This controlled cooling is the determining factor in producing nanoparticles of uniform size.

The Physics of Condensation
Kinetic Energy Transfer
The synthesis begins with an evaporation heat source converting raw materials into a highly energetic vapor. Upon entering the helium-filled chamber, these metal atoms possess significant kinetic energy.
The Role of Collision
Cooling occurs through direct physical interaction. The metal vapor atoms collide with the cooler helium atoms filling the chamber.
Rapid Deceleration
Each collision transfers energy from the metal vapor to the helium. This results in a drastic and immediate loss of kinetic energy for the metal atoms, effectively lowering their temperature.
Controlling Particle Formation
Triggering Nucleation
As the metal atoms lose energy, they can no longer maintain a gaseous state. This energy drop forces the atoms to bond, triggering nucleation where atoms cluster together to form solids.
Ensuring Uniform Growth
Because the helium environment surrounds the vapor, the cooling process is distributed evenly. This mechanism allows for the controlled growth of the nuclei.
Resulting Uniformity
The ultimate output of this specific cooling interaction is consistency. The process yields nanoparticles that are uniform in size, rather than a mixture of random clusters.
Understanding the Constraints
Requirement for Purity
The reference specifies the use of pure helium. Because the goal is physical condensation rather than chemical reaction, contaminants in the gas could alter the composition of the final product.
The Necessity of Balance
The process relies on a specific interaction rate between the vapor and the gas. If the energy transfer is not managed correctly via the helium medium, the control over nucleation and particle size is lost.
Optimizing the Synthesis Process
To effectively utilize inert gas condensation, you must view helium not just as a filler gas, but as an active thermal component.
- If your primary focus is high uniformity: Maintain strictly pure helium levels to ensure collisions result only in physical cooling, not chemical alteration.
- If your primary focus is particle formation: Regulate the interaction between the vapor and the helium to ensure the loss of kinetic energy is rapid enough to trigger immediate condensation.
Mastering the helium environment is the key to transforming volatile vapor into precise nanostructures.
Summary Table:
| Feature | Role of Helium in Synthesis |
|---|---|
| Primary Function | Energy dissipation & thermal braking |
| Mechanism | Kinetic energy transfer via physical collisions |
| Effect on Vapor | Rapid deceleration and cooling |
| Final Outcome | Controlled nucleation and uniform particle size |
| Requirement | High purity to prevent chemical contamination |
Elevate Your Nanomaterial Synthesis with KINTEK
Precise nanoparticle production requires perfectly controlled environments. KINTEK provides industry-leading high-temperature lab furnaces, including Vacuum, CVD, and customizable systems, designed to facilitate advanced methods like inert gas condensation.
Backed by expert R&D and precision manufacturing, our systems ensure the thermal stability and gas purity your research demands. Whether you need a standard Muffle furnace or a specialized Rotary system, KINTEK delivers the tools to transform volatile vapors into precise nanostructures.
Contact our specialists today to discuss your custom furnace needs!
Visual Guide
References
- “Pharmaceutical Nanoparticles: Detailed Review of Types, Preparation Methods, and Applications”. DOI: 10.35629/4494-100221922223
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Spark Plasma Sintering SPS Furnace
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
People Also Ask
- Why is it necessary to use an annealing furnace at 350°C for three hours? Ensuring Glass Stability and Clarity
- What is the purpose of using controlled anaerobic environments for peat carbonization? Unlock High-Energy Industrial Fuel
- What is the purpose of equipping the condensation section of a sodium heat pipe with a specialized insulation cover?
- Why is an air-ventilated oven necessary for GFPP surface modification? Achieve Maximum Solar Reflectance
- What is the mechanism of the thermal reduction process for graphene oxide-cement? Master Thermal Activation in Furnaces
- What are the advantages of using a vacuum low-temperature microwave-assisted pyrolysis system for LCP? (Enhanced Guide)
- What causes the increase in specific gravity of Moso Bamboo? Master Cellular Densification in Heat Treatment
- What is the role of a precision heating system in HEA synthesis? Achieve Atomic Uniformity at 220 °C



















