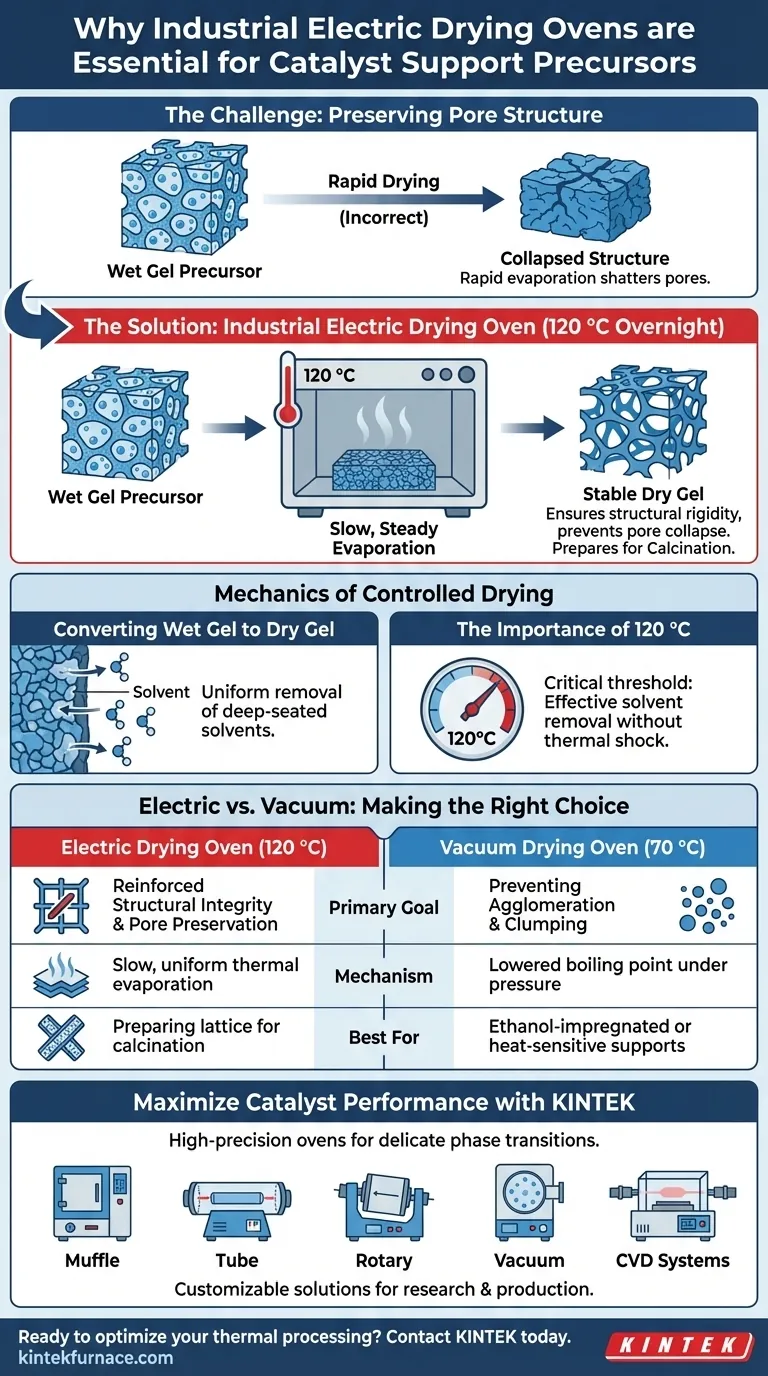
An industrial electric drying oven is strictly required to ensure the structural survival of the catalyst support. By maintaining a steady temperature of 120 °C over a long duration, it facilitates the slow, thorough removal of water and volatile solvents. This controlled environment converts wet gel into dry gel while preventing the rapid evaporation that commonly leads to pore collapse.
The primary function of overnight treatment in an electric oven is the preservation of the precursor's internal architecture. By ensuring slow, steady evaporation, the process safeguards the pore network, establishing the necessary physical stability for subsequent high-temperature calcination.

The Mechanics of Controlled Drying
Converting Wet Gel to Dry Gel
The fundamental goal of this stage is the phase transition of the precursor material. The oven facilitates the complete removal of liquids trapped within the gel network.
This is not merely about drying a surface; it is about extracting solvents from deep within the material's matrix. A long-duration cycle ensures that this removal is uniform throughout the bulk of the material, effectively turning a fragile "wet gel" into a stable "dry gel."
The Importance of 120 °C
The specific temperature setting of 120 °C acts as a critical threshold. It is high enough to drive off water and standard solvents effectively but controlled enough to avoid thermal shock.
Maintaining this temperature overnight guarantees that the drying is thorough. It eliminates residual moisture that could cause destructive steam pockets during later heating stages.
Preserving Pore Architecture
Preventing Structural Collapse
The speed of drying is just as important as the final dryness. If a precursor is heated too rapidly, the solvents inside vaporize explosively on a microscopic scale.
This rapid expansion can shatter the delicate walls of the pore structure. The industrial electric oven provides a slow thermal ramp, allowing vapors to escape gently without crushing the internal framework of the support.
Establishing a Foundation for Calcination
This drying step is a prerequisite for the more aggressive calcination process that follows. If the precursor enters the calcination phase with a compromised pore structure or trapped moisture, the final catalyst will be defective.
The electric oven ensures the "skeleton" of the material is rigid and void of volatiles before it faces high-temperature treatment.
Understanding the Trade-offs: Electric vs. Vacuum
The Standard Electric Approach
The industrial electric oven described above is ideal when the priority is structural rigidity and deep pore preservation for standard precursors. It excels at robust, long-duration heating at moderate temperatures (120 °C).
The Vacuum Alternative
It is important to distinguish this from vacuum drying, which serves a slightly different purpose. A vacuum oven is typically used at lower temperatures (e.g., 70 °C), particularly for ethanol-impregnated gels or carbon supports.
While the electric oven focuses on structural hardening, the vacuum oven focuses on preventing agglomeration and protecting heat-sensitive chemical components by lowering the boiling point of solvents. Choosing the wrong oven type can lead to either incomplete drying (too cool) or particle clumping (lack of vacuum).
Making the Right Choice for Your Goal
To ensure optimal catalyst performance, select your drying method based on the specific stability requirements of your precursor:
- If your primary focus is Structural Integrity: Use the industrial electric oven at 120 °C to prevent pore collapse and prepare the lattice for calcination.
- If your primary focus is Preventing Agglomeration: Consider a vacuum drying oven at lower temperatures (e.g., 70 °C) to keep powders loose and chemically stable.
The correct drying protocol does not just remove water; it defines the final geometry and effectiveness of your catalyst.
Summary Table:
| Feature | Electric Drying Oven (120 °C) | Vacuum Drying Oven (70 °C) |
|---|---|---|
| Primary Goal | Structural integrity & pore preservation | Preventing agglomeration & clumping |
| Mechanism | Slow, uniform thermal evaporation | Lowered boiling point under pressure |
| Key Outcome | Converts wet gel to stable dry gel | Protects heat-sensitive components |
| Best Used For | Preparing lattice for calcination | Ethanol-impregnated or carbon supports |
Maximize Your Catalyst Performance with KINTEK
Don't let rapid evaporation compromise your material's architecture. KINTEK provides high-precision industrial electric and vacuum drying ovens designed specifically for the delicate phase transitions required in catalyst preparation.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temp furnaces, all customizable for your unique research or production needs. Ensure your precursors achieve the structural rigidity they need before calcination.
Ready to optimize your thermal processing? Contact KINTEK today to consult with our experts on the ideal solution for your laboratory.
Visual Guide
References
- Jintao Miao, Jing Zhou. Effect of Ti dopants in Ce <sub> 1− <i>x</i> </sub> Ti <sub> <i>x</i> </sub> O <sub> 2− <i>δ</i> </sub> -supported Ni catalysts: structure, redox properties, and carbon resistance in DRM. DOI: 10.1039/d5cy00760g
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Kiln for Pyrolysis Plant Heating
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What technical requirements are placed on heating equipment for fast pyrolysis? Master High-Yield Bio-Oil Production
- What is the primary function of the low-temperature pyrolysis process? Ensure Safe Battery Recycling with Pretreatment
- What is the role of sintering in CsPbBr3-SiO2 preparation? Unlock Ultra-Stability with Precise Thermal Sealing
- What is the purpose of preheating reinforcement particles? Optimize AMC Stir Casting Results
- What role does pack media play in the solid-state powder boriding process? Enhance Metal Hardness at High Temperatures
- Why are precision hydrothermal reactors necessary for nut shell modification? Unlock Biomass Energy Potential
- Why is a high-precision furnace critical for refractory castables? Ensure Structural Integrity & Mineral Stability
- What is the function of a laboratory oven in ZnO processing? Optimize Precursor Drying & Prevent Agglomeration



















