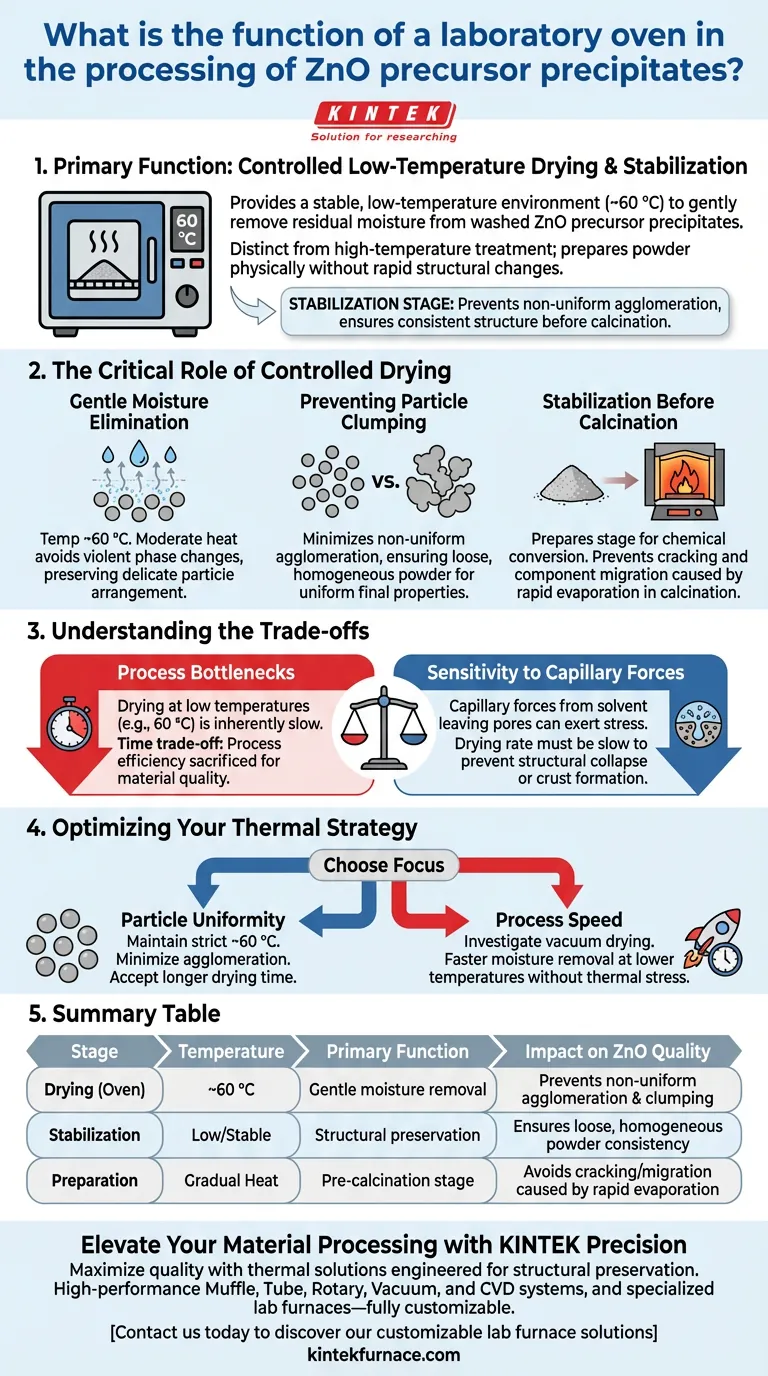
The primary function of a laboratory oven in this context is to provide a stable, low-temperature environment—typically around 60 °C—to gently remove residual moisture from washed Zinc Oxide (ZnO) precursor precipitates. This controlled drying phase is distinct from high-temperature treatment; its specific purpose is to prepare the powder physically without inducing rapid structural changes.
The laboratory oven acts as a stabilization stage, preventing non-uniform agglomeration of the powder. By removing moisture slowly, it ensures the precursor material maintains a consistent structure before undergoing the more aggressive calcination process.

The Critical Role of Controlled Drying
The transition from a wet chemical precipitate to a solid oxide requires careful thermal management. The laboratory oven bridges the gap between the washing stage and the final heat treatment.
Gentle Moisture Elimination
The key operational parameter for ZnO precursors is the temperature, often set near 60 °C.
At this moderate heat, water is evaporated at a rate that avoids violent phase changes. This "gentle" approach is vital for preserving the delicate arrangement of the precipitate particles.
Preventing Particle Clumping
If moisture is removed too aggressively, particles tend to stick together unevenly.
The oven minimizes non-uniform agglomeration, ensuring that the powder remains loose and homogeneous. This physical consistency is crucial for ensuring the final ZnO product has uniform properties.
Stabilization Before Calcination
The oven does not perform the final chemical conversion; it prepares the stage for it.
By delivering a thoroughly dried precursor to the calcination furnace, the oven prevents issues caused by rapid moisture evaporation, such as particle cracking or component migration, which can occur if wet materials are exposed to high heat immediately.
Understanding the Trade-offs
While the laboratory oven is essential for quality control, it introduces specific constraints to the processing workflow that must be managed.
Process Bottlenecks
Drying at low temperatures (e.g., 60 °C) is inherently slow.
This creates a time trade-off where process efficiency is sacrificed for material quality. Attempting to speed up this step by raising the temperature significantly risks triggering the very agglomeration issues the oven is meant to prevent.
Sensitivity to Capillary Forces
Even within an oven, the physics of drying can affect the material.
As solvent leaves the pores of a material, capillary forces can exert stress on the particle structure. While this is more critical in impregnated catalyst supports, it remains a factor here: the drying rate must be slow enough to prevent structural collapse or "crust" formation on the precipitate surface.
Optimizing Your Thermal Strategy
To ensure high-quality ZnO production, you must balance the need for dry material with the preservation of particle morphology.
- If your primary focus is particle uniformity: Maintain the oven temperature strictly around 60 °C to minimize agglomeration, accepting the longer drying time as a necessary cost.
- If your primary focus is process speed: Investigate vacuum drying options, which may allow for faster moisture removal at lower temperatures without the thermal stress of higher heat.
Ultimately, the laboratory oven is not just a heating device, but a tool for structural preservation, ensuring your precursor is physically ready for the chemical transformation of calcination.
Summary Table:
| Stage | Temperature | Primary Function | Impact on ZnO Quality |
|---|---|---|---|
| Drying (Oven) | ~60 °C | Gentle moisture removal | Prevents non-uniform agglomeration & clumping |
| Stabilization | Low/Stable | Structural preservation | Ensures loose, homogeneous powder consistency |
| Preparation | Gradual Heat | Pre-calcination stage | Avoids cracking/migration caused by rapid evaporation |
Elevate Your Material Processing with KINTEK Precision
Maximize the quality of your ZnO precursors with thermal solutions engineered for structural preservation. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to meet your unique research and production needs.
Don't let poor thermal management compromise your particle morphology. Our expert team is ready to help you find the perfect balance between process speed and material uniformity.
Contact us today to discover our customizable lab furnace solutions
Visual Guide
References
- Zhenchao Sun, Pengfei Cheng. Gas Sensor for Efficient Acetone Detection and Application Based on Au-Modified ZnO Porous Nanofoam. DOI: 10.3390/s24248100
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the characteristics of a Batch Reactor for plastic pyrolysis? A Guide to Versatile Waste Processing
- What is the operational mechanism of a smelting reduction furnace (SRF)? Optimize Your HAlMan Metallurgy Process
- Why is specialized dewaxing and annealing necessary for glass-to-metal seals? Ensure Hermeticity and Clarity
- How does a precision carbon dioxide gas flow control system influence the precipitation of high-purity lithium carbonate?
- Why is a high-purity argon flow control system essential? Ensure Precision in Metallurgy Simulations
- What is an industrial oven and which industries use it? Discover Versatile Thermal Processing Solutions
- How does a laboratory furnace work? Master the Heating Mechanisms for Your Lab
- Why are deoxidizer powders sealed inside iron bolts? Achieve Precise Chemical Control in Steel Inclusion Preparation



















