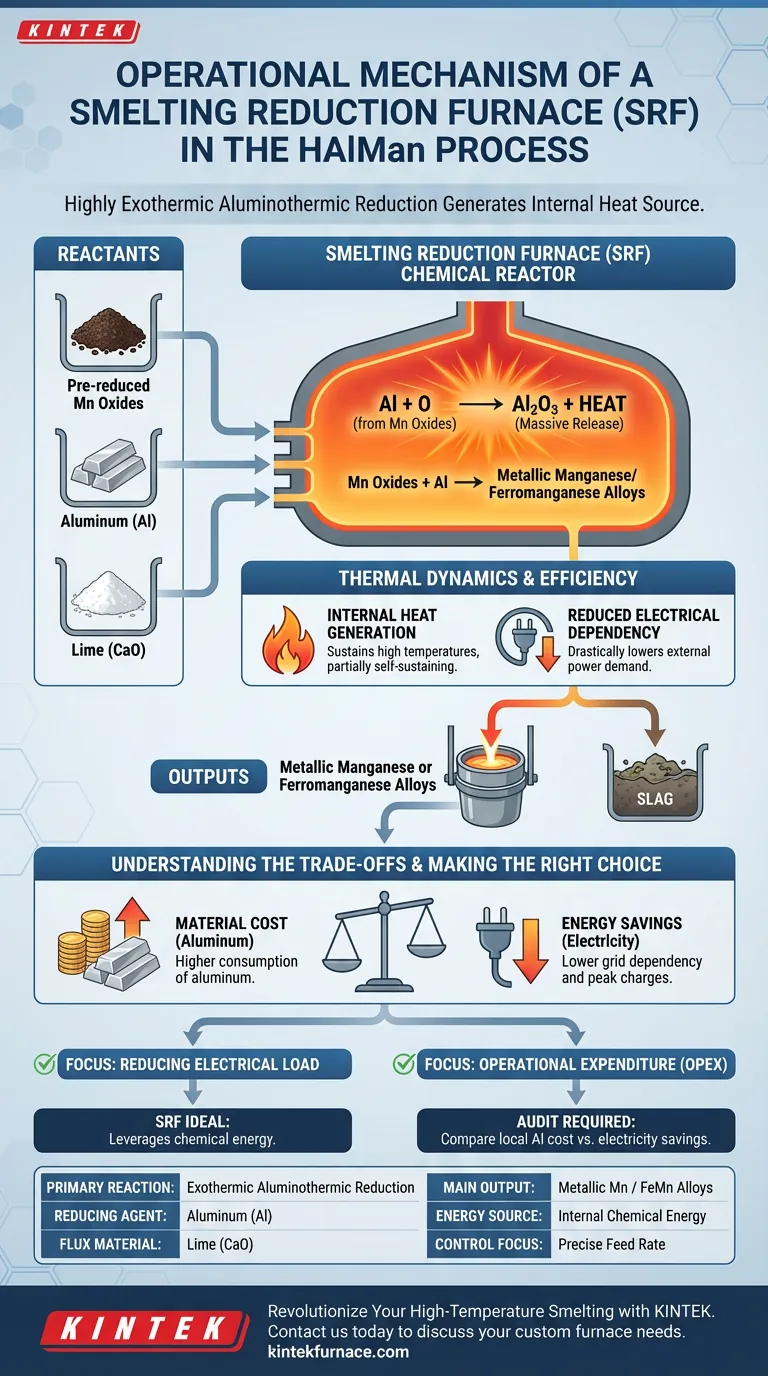
The operational mechanism of the Smelting Reduction Furnace (SRF) centers on a highly exothermic aluminothermic reduction reaction. By introducing aluminum and lime to pre-reduced manganese oxides, the furnace converts these oxides into metallic manganese or ferromanganese alloys while generating its own internal heat source.
The SRF differentiates itself by utilizing the chemical energy released during aluminum oxidation to drive the smelting process. This approach transforms the furnace into a chemical reactor that sustains its own high temperatures, significantly reducing the reliance on external electrical power.

The Mechanics of Aluminothermic Reduction
The Role of Reactants
The process begins with the precise addition of aluminum and lime to the furnace containing pre-reduced manganese oxides.
The aluminum acts as the primary reducing agent, stripping oxygen from the manganese oxides. The lime serves as a flux, likely aiding in slag formation and managing impurities during the separation of the metal.
Chemical Energy Release
The core driver of this mechanism is the reaction between aluminum and oxygen.
This interaction is intensely exothermic, meaning it releases a massive amount of chemical energy in the form of heat. This energy release is not merely a byproduct; it is the primary engine for maintaining the thermal environment inside the vessel.
Transformation to Alloy
Under these high-temperature conditions, the chemical bonds holding the manganese oxides together are broken.
The result is the full reduction of the oxides into metallic manganese or, depending on the specific inputs, ferromanganese alloys. This liquid metal settles at the bottom of the furnace for tapping.
Thermal Dynamics and Efficiency
Internal Heat Generation
Unlike traditional furnaces that rely heavily on electric arcs or induction for heat, the SRF leverages the reaction itself.
The heat generated by the aluminothermic reaction is sufficient to maintain the high temperatures required for smelting. This effectively makes the process partially self-sustaining from a thermal perspective.
Reduced Electrical Dependency
Because the chemical reaction provides a substantial portion of the necessary thermal energy, the demand for external electrical power is drastically lowered.
This operational shift allows the facility to decouple production costs from volatile electricity market prices, relying instead on the chemical potential of the input materials.
Understanding the Trade-offs
While the thermodynamic benefits are clear, this mechanism introduces specific operational considerations regarding input costs.
Material Cost vs. Energy Savings
The primary trade-off in this mechanism is the exchange of electrical cost for material cost.
While you save significantly on electricity, the process requires continuous consumption of aluminum, which is generally a more expensive commodity than carbon-based reductants. The economic viability of the SRF depends on the price spread between industrial electricity and aluminum.
Process Control
Aluminothermic reactions are rapid and intense.
Operators must maintain precise control over the feed rate of aluminum and lime to prevent thermal runaways or incomplete reduction, demanding rigorous process monitoring compared to slower, electrically heated methods.
Making the Right Choice for Your Goal
When evaluating the HAlMan process SRF for your operations, consider your primary resource constraints.
- If your primary focus is reducing electrical load: The SRF is ideal as it leverages chemical energy to minimize grid dependency and peak power charges.
- If your primary focus is operational expenditure (OPEX): You must carefully audit the local cost of aluminum supply against the projected savings in electricity to ensure a positive margin.
The SRF represents a shift from electrically driven metallurgy to chemically driven thermodynamics, offering high thermal efficiency for operators with access to cost-effective aluminum.
Summary Table:
| Feature | SRF Operational Detail |
|---|---|
| Primary Reaction | Exothermic Aluminothermic Reduction |
| Reducing Agent | Aluminum (Al) |
| Flux Material | Lime (CaO) for slag management |
| Main Output | Metallic Manganese or Ferromanganese Alloys |
| Energy Source | Internal chemical energy (reduces electrical dependency) |
| Control Focus | Precise feed rate to manage rapid thermal release |
Revolutionize Your High-Temperature Smelting with KINTEK
Maximize your metallurgical efficiency and reduce grid dependency with advanced furnace technology. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to your specific HAlMan process or smelting requirements.
Whether you are scaling up aluminothermic reduction or need precise thermal control for alloy development, our engineering team is ready to deliver the solution you need. Contact us today to discuss your custom furnace needs and optimize your production performance.
Visual Guide
References
- Lu, Shao-Lun, Max-Planck-Institut für Nachhaltige Materialien. Making High Mn Steel by Sustainable Ferromanganese Pre-alloy for Cryogenic Applications. DOI: 10.5281/zenodo.17520990
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- Why is an industrial constant temperature oven required to cure thermal pads? Ensure Superior Polymer Cross-Linking
- How does a Zinc Oxide (ZnO) catalyst affect PET pyrolysis? Optimize Yields & Efficiency
- Why is precise sample loading critical in CO2 capture experiments? Avoid Bed Effects and Ensure Data Integrity
- What is the function of a pure graphite sheet within a microwave hybrid heating setup? Ensure Pure Ni-BN Cladding
- How does Thermogravimetric Analysis (TGA/DTG) provide industrial guidance? Optimize Blast Furnace Dust Treatment
- Why is an auxiliary gas supply device required for oil sludge pyrolysis? Ensure Stable Thermal Balance
- What are the advantages of a multimode microwave furnace? Accelerate B-doped SiC Nanowire Synthesis for Higher Yields
- Why is a constant temperature drying oven used for activated carbon? Ensure Pore Integrity and Adsorption Efficiency



















