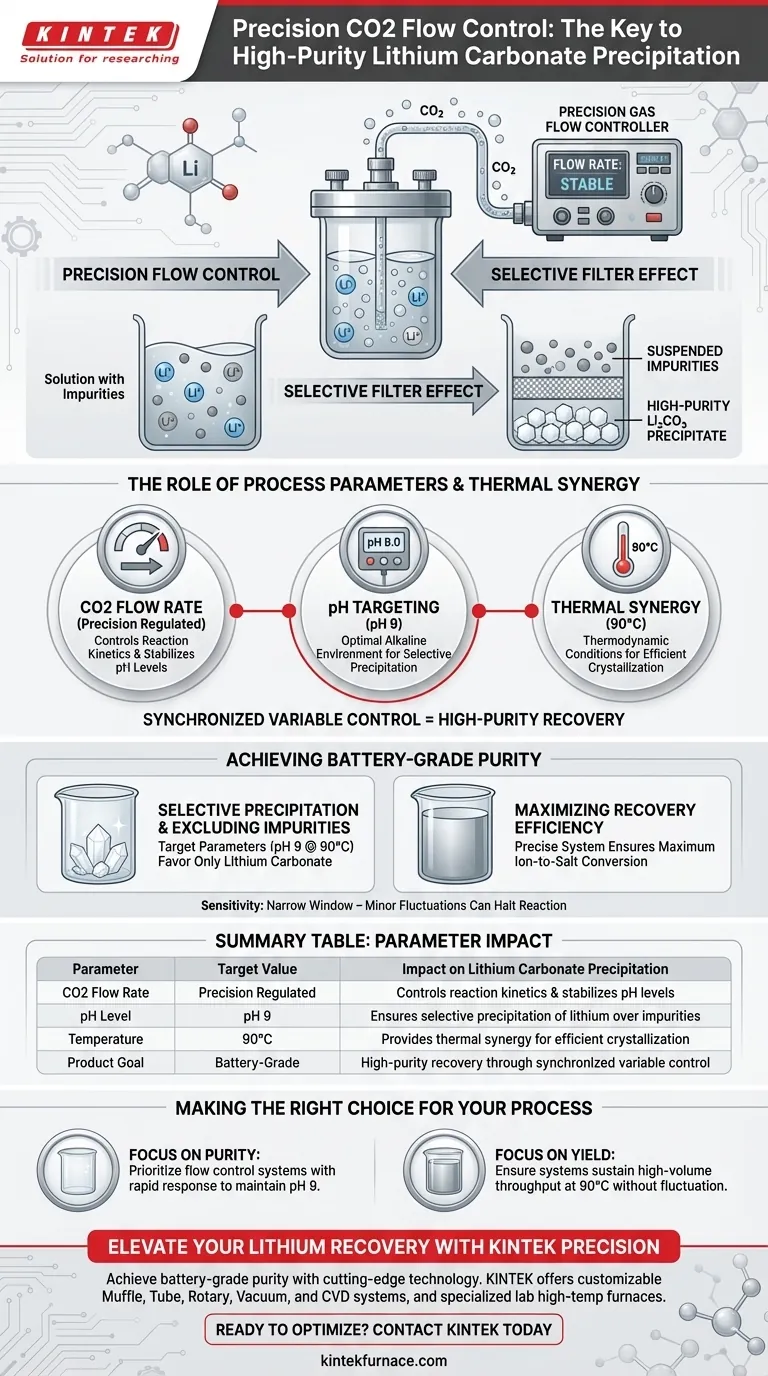
A precision carbon dioxide gas flow control system serves as the critical lever for regulating reaction kinetics during lithium recovery. By strictly modulating the rate of CO2 input, the system works in tandem with thermal and pH controls to force the specific combination of lithium ions with carbonate ions. This targeted modulation is what allows operators to transition from basic chemical mixing to the high-yield production of battery-grade materials.
Precision flow control transforms the precipitation process into a selective filter, ensuring lithium forms a solid precipitate while unwanted impurities remain suspended in the solution.

The Role of Process Parameters
Regulating Reaction Conditions
The primary function of the gas flow system is to maintain a stable chemical environment. By controlling the exact volume and speed of CO2 introduction, the system dictates how available lithium ions interact with the carbonate source.
The Importance of pH Targeting
To achieve successful precipitation, the system typically targets a specific alkalinity, often maintaining a pH of 9. The CO2 flow acts as a regulator to stabilize this pH level, preventing the solution from becoming too acidic or too basic for the desired reaction.
Thermal Synergy
Gas flow control does not operate in a vacuum; it functions alongside strict temperature regulation, generally around 90°C. This combination of precise gas input and high temperature creates the thermodynamic conditions necessary for efficient crystallization.
Achieving Battery-Grade Purity
Selective Precipitation
The ultimate goal of precision control is differentiation. By holding the reaction at exact parameters (pH 9 at 90°C), the system ensures that only lithium carbonate precipitates out of the solution.
Excluding Impurities
Conditions that favor lithium precipitation often differ from those required for other dissolved contaminants. Precision control prevents the co-precipitation of these impurities, resulting in a high-purity, battery-grade final product.
Maximizing Recovery Efficiency
Beyond purity, the system drives total yield. An erratic flow rate can lead to incomplete reactions, but a precise system ensures the maximum amount of lithium is recovered from the solution as a salt.
Understanding the Constraints
Sensitivity to Deviation
The window for high-purity precipitation is narrow. Even minor fluctuations in CO2 flow can shift the pH away from the optimal target of 9, potentially halting the reaction or allowing impurities to contaminate the batch.
Interdependency of Variables
Success relies on the synchronization of all three variables: flow rate, pH, and temperature. If the temperature drops below 90°C, a perfect CO2 flow rate may still fail to produce the desired efficiency, requiring a holistic approach to system management.
Making the Right Choice for Your Process
Optimizing your precipitation circuit requires aligning your control strategy with your specific output goals.
- If your primary focus is Purity: Prioritize flow control systems that offer rapid response times to maintain pH 9, ensuring the exclusion of impurities.
- If your primary focus is Yield: Ensure your system can sustain high-volume throughput at 90°C without fluctuating, maximizing the conversion of ions to salts.
True process control is achieved when gas flow, temperature, and pH operate as a single, synchronized unit.
Summary Table:
| Parameter | Target Value | Impact on Lithium Carbonate Precipitation |
|---|---|---|
| CO2 Flow Rate | Precision Regulated | Controls reaction kinetics and stabilizes pH levels. |
| pH Level | pH 9 | Ensures selective precipitation of lithium over impurities. |
| Temperature | 90°C | Provides thermal synergy for efficient crystallization. |
| Product Goal | Battery-Grade | High-purity recovery through synchronized variable control. |
Elevate Your Lithium Recovery with KINTEK Precision
Achieving battery-grade purity requires more than just mixing—it demands absolute control over your thermal and chemical environment. KINTEK provides the cutting-edge technology needed to synchronize your precipitation process.
Backed by expert R&D and world-class manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside specialized lab high-temp furnaces—all fully customizable to meet your unique lithium processing needs. Whether you are focusing on maximizing yield or ensuring ultra-high purity, our systems provide the stability and precision your lab requires.
Ready to optimize your high-purity production? Contact KINTEK today to discuss your custom solution!
Visual Guide
References
- Sara El Hakim, Alexandre Chagnes. A Novel Approach to Lithium Extraction From Spodumene by Combining Maleic Acid Leaching and Cyanex 936P Solvent Extraction. DOI: 10.1002/metm.70011
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- Why is an electric blast drying oven required for CRP microstructure analysis? Ensure Data Accuracy with Proper Drying
- How does zinc chloride (ZnCl2) serve as a structural template? Engineering High-Porosity Nitrogen-Doped Carbon
- How does Thermogravimetric Analysis (TGA/DTG) provide industrial guidance? Optimize Blast Furnace Dust Treatment
- How does the carbon reductant ratio influence the selective reduction of ferronickel? Mastering Alloy Purity
- What is the importance of defining accurate heat transfer coefficients for slag? Master Thermal Stress Prediction
- What role does an industrial-grade POCl3 diffusion furnace system play in DOSS? Master Quantitative Phosphorus Control
- How do lab high-temp furnaces and air quenching coordinate in o-LISO synthesis? Master the Thermal Transition
- How does a precision temperature-controlled heating furnace enhance medium-entropy alloys? Achieve Optimal Hardness



















