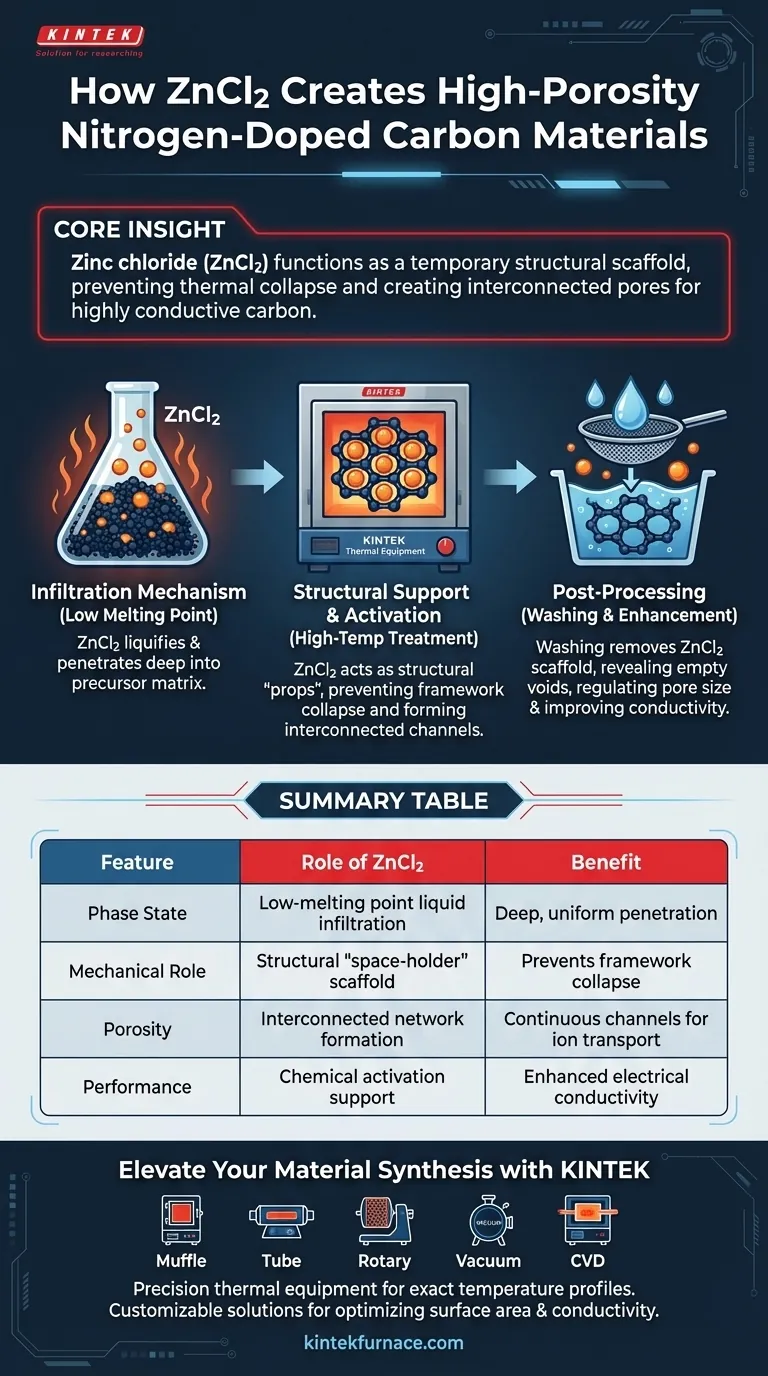
Zinc chloride (ZnCl2) functions as a temporary structural scaffold during the synthesis of nitrogen-doped carbon materials. Its primary role is to penetrate the precursor material in a liquid state, physically preventing the carbon framework from collapsing under high heat, and creating a network of interconnected pores once the salt is washed away.
Core Insight: Thermal processing typically causes carbon precursors to shrink and lose porosity. ZnCl2 acts as a "space-holder" that maintains the material's volume during heating, resulting in a highly conductive structure with a precisely regulated pore distribution after the salt is removed.

The Mechanism of Infiltration
Leveraging Low Melting Points
The effectiveness of ZnCl2 stems from its low melting point. Because it liquifies easily, it transforms into a fluid state early in the thermal process.
Deep Precursor Penetration
Once molten, the ZnCl2 acts as a solvent that penetrates deep into the carbon precursor matrix. This ensures the agent is distributed evenly throughout the material, rather than just coating the surface.
Structural Support and Activation
Preventing Framework Collapse
During high-temperature treatment, carbon materials naturally tend to densify and collapse. ZnCl2 acts as a structural prop, occupying internal space and mechanically supporting the carbon framework to keep it expanded.
Creating Interconnected Channels
Because the ZnCl2 is distributed throughout the material, it forms a continuous network within the carbon. This "skeleton" ensures that the voids created are interconnected rather than isolated bubbles.
Post-Processing and Material Enhancement
Formation via Removal
The final porous structure is revealed only after the thermal process is complete. The ZnCl2 is removed through subsequent washing, leaving behind the empty channels where the salt used to reside.
Regulating Pore Size
This method is not random; it allows for high precision. By using ZnCl2, engineers can effectively regulate the pore size distribution, tailoring the material to specific requirements.
Improving Electrical Conductivity
Beyond just creating space, this activation method enhances the material's performance. The resulting nitrogen-doped carbon exhibits improved electrical conductivity, making it suitable for advanced electronic applications.
Process Considerations and Trade-offs
The Requirement of Post-Treatment
While ZnCl2 is an effective template, it is not a "one-step" additive. The process strictly requires a washing phase to remove the salt scaffold; without this step, the pores remain blocked and the material cannot function as intended.
Making the Right Choice for Your Goal
When designing a synthesis protocol for nitrogen-doped carbon, consider your specific performance targets:
- If your primary focus is surface area optimization: Utilize ZnCl2 for its ability to prevent collapse and create deep, interconnected pore channels.
- If your primary focus is electronic performance: Rely on this activation method to specifically improve the electrical conductivity of the final carbon material.
By using ZnCl2 as a sacrificial scaffold, you convert the risk of thermal collapse into an opportunity for precise structural engineering.
Summary Table:
| Feature | Role of ZnCl2 in Synthesis | Benefit to Carbon Material |
|---|---|---|
| Phase State | Low-melting point liquid infiltration | Deep, uniform penetration of precursors |
| Mechanical Role | Structural "space-holder" scaffold | Prevents framework collapse during heating |
| Porosity | Interconnected network formation | Creates continuous channels for ion transport |
| Final Step | Post-process salt removal (washing) | Reveals high surface area & regulated pore size |
| Performance | Chemical activation/doping support | Enhanced electrical conductivity & conductivity |
Elevate Your Material Synthesis with KINTEK
Precision in thermal processing is critical when utilizing ZnCl2 templates for advanced carbon materials. KINTEK provides the high-performance thermal equipment necessary to achieve the exact temperature profiles required for successful infiltration and activation.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique lab high-temp furnace needs. Whether you are optimizing surface area or enhancing electrical conductivity for nitrogen-doped carbon, our solutions ensure consistent, repeatable results.
Ready to optimize your carbon synthesis? Contact KINTEK today for a custom solution!
Visual Guide
References
- Xing Huang, Dessie Ashagrie Tafere. Waste-derived green N-doped materials: mechanistic insights, synthesis, and comprehensive evaluation. DOI: 10.1039/d5su00555h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Magnesium Extraction and Purification Condensing Tube Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
People Also Ask
- What are the benefits of using electric actuators in this solution? Achieve Precision, Safety, and Efficiency in Automation
- What is the purpose of maintaining a 70°C environment in Li-NASICON experiments? Accelerate Your Battery Research
- Why is 10^-6 mbar pressure required for CZTS PLD? Ensure Pure, High-Efficiency Thin Film Deposition
- Why is a vacuum system composed of molecular and mechanical pumps essential? Ensure Purity in Magnetron Sputtering
- What is the significance of temperature control precision in high-temperature furnaces for carbon-doped titanium dioxide?
- What is the purpose of post-treating Nitrogen-doped Carbide-Derived Carbon (N-CDC)? Optimize Purity & Performance
- What is the purpose of coating aluminum electrodes with Au80Pd20? Enhancing Precision in Nanoparticle Characterization
- How does a bias power supply influence AlCrSiWN coatings? Master Ion Bombardment for Superior Durability



















