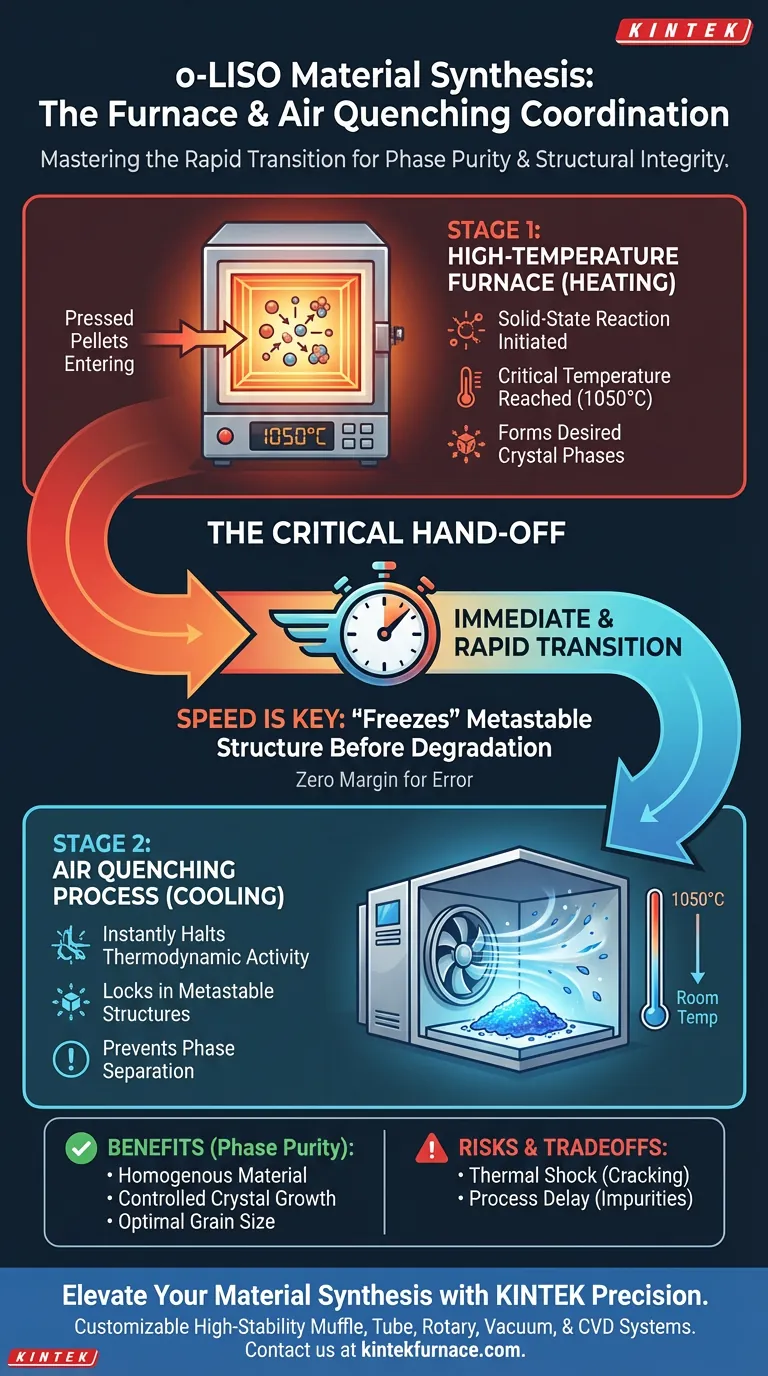
The coordination between laboratory high-temperature furnaces and air quenching processes is defined by a precise, rapid transition from extreme heat to ambient conditions. During the pre-calcination stage of o-LISO synthesis, the furnace drives the solid-state reaction at 1050°C, while the subsequent air quenching instantly halts thermodynamic activity to preserve the material's structural integrity.
Core Takeaway The success of o-LISO synthesis relies on the speed of the "hand-off" between heating and cooling. The high-temperature furnace creates the necessary crystal phase, but only immediate air quenching can "freeze" this metastable structure before it degrades into unwanted phases.
The Role of the High-Temperature Furnace
Initiating the Solid-State Reaction
The process begins with pressed pellets entering a laboratory high-temperature furnace. The primary objective here is to supply sufficient thermal energy to trigger a solid-state reaction.
Reaching the Critical Temperature
The furnace must maintain a stable temperature of 1050°C. At this specific thermal plateau, the precursor materials begin to reorganize, initiating the formation of the desired crystal phases.
The Mechanism of Air Quenching
The Immediate Transition
The coordination between the furnace and the quenching step is strictly temporal. As soon as the heating cycle concludes, the material is immediately subjected to air quenching.
Rapid Thermal Drop
This process forces the material to cool from 1050°C down to room temperature in a very short timeframe. The speed of this temperature drop is the single most critical variable in this stage of synthesis.
Why This Coordination is Critical
Locking in Metastable Structures
The high temperature creates a specific, desired crystal structure that is often metastable. If the material is allowed to cool slowly, it may naturally revert to a more thermodynamically stable—but functionally inferior—state.
Preventing Phase Separation
Rapid cooling denies the atoms the time they need to diffuse and rearrange. This effectively blocks phase separation, ensuring the material remains homogenous.
Controlling Crystal Growth
Extended exposure to high heat during a slow cooling process promotes excessive crystal growth. Air quenching arrests this growth instantly, preserving the optimal grain size established during the heating phase.
Understanding the Trade-offs
The Risk of Thermal Shock
While rapid cooling is essential for phase purity, it introduces significant thermal stress. If the pellets are not pressed correctly or if the quench is uneven, the material may crack or shatter due to the sudden contraction.
The Danger of Process Delay
The "hand-off" allows for zero margin for error. Even a brief delay between removing the material from the furnace and initiating the airflow allows the temperature to drop slowly, potentially introducing impurities or phase degradation that the quench was meant to prevent.
Ensuring Process Integrity
To maximize the quality of your o-LISO material, you must balance the intensity of the heat with the speed of the cooling.
- If your primary focus is Phase Purity: Ensure the transfer from the furnace to the quenching zone is instantaneous to prevent thermodynamic relaxation.
- If your primary focus is Structural Integrity: Verify pellet density prior to heating to minimize the risk of mechanical failure during the thermal shock of quenching.
Mastering this thermal transition is the key to synthesizing high-performance o-LISO materials.
Summary Table:
| Process Stage | Key Parameters | Primary Objective | Critical Success Factor |
|---|---|---|---|
| Pre-calcination | 1050°C Stable | Solid-state reaction & phase formation | Uniform thermal energy supply |
| Air Quenching | 1050°C to Room Temp | "Freeze" metastable structures | Immediate transition speed |
| Integration | Instantaneous Hand-off | Prevent phase separation & grain growth | Minimizing thermal relaxation time |
Elevate Your Material Synthesis with KINTEK Precision
High-performance o-LISO materials require the perfect balance of intense heat and rapid cooling. KINTEK provides the specialized equipment needed to master this transition. Backed by expert R&D and manufacturing, we offer high-stability Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your specific lab requirements.
Don't let process delays compromise your crystal phase purity. Ensure structural integrity and phase excellence with our advanced laboratory high-temperature furnaces.
Contact KINTEK today to discuss your custom furnace needs and see how our technology brings precision to your research.
Visual Guide
References
- Yu Chen, Gerbrand Ceder. Unlocking Li superionic conductivity in face-centred cubic oxides via face-sharing configurations. DOI: 10.1038/s41563-024-01800-8
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is it necessary to dry Industrial EAF slag before hydrogen reduction? Crucial Safety and Accuracy Prep
- What is the purpose of using a preheated stainless steel plate when processing molten glass? Avoid Thermal Stress.
- What is the importance of dynamic sealing in an InP crystal growth furnace? Ensure Pressure Integrity & Motion Control
- What is the necessity of using a laboratory vacuum drying oven? Preserving Porous Carbon Integrity
- Why is a constant temperature blast drying oven necessary for biomass carbon impregnation? Optimize Material Structure
- What is the primary role of high-purity hydrogen in heat treatment? Achieve Superior Metallic Coating Protection
- How does the perpendicular orientation of substrate holders benefit VTD? Maximize Efficiency and Thermal Control
- How are thermal processing equipment commonly categorized? Choose the Right Furnace for Your Lab



















